PHOTOSYNTHESIS
The free chemical energy required for life processes is derived from organic food materials by a process called Biological Oxidation. If organisms are allowed to utilize the existing food materials on this planet, it will be exhausted in short period of time. However, nature has devised a unique mechanism by means of which it replenishes the organic food materials in excess and this is achieved by harnessing the solar energy by plants.

Light to product
Definition: Photosynthesis can be defined as a “series of processes in which solar electro magnetic radiation energy (SEMR) is captured, converted and conserved in the form of chemical energy by green plants”. Biochemically, this process can also be defined “as light sensitized Red-Ox process” for it involves light induced Oxidation-Reduction processes.
Solar Energy
Sun emits light in the form of solar electro magnetic radiation (SEMR) of which only 40% reaches the surface of earth and rest is absorbed by O2, O3, CO2, dust and H2O present in outer atmosphere of the earth. Out of this only 1% is used by land plants which form about 15% of total plant population, and oceanic plants (about 85%) in fixing a total of 35-146 billion tones of carbon every year which is equivalent to 1.8-13 x 1012 K. Cals, of chemical energy.
SEMR Spectrum: The SEMR spectrum is quite large, consisting of cosmic rays at one extremity and radio waves at the other, of which, plants use only blue and red parts of the visible light for photosynthesis. Light travels in waves, in the form of discrete energy packets called photons. The energy content of each photon varies and depends upon the frequency and speed of the light. For example, light with shorter wavelength has more energy than the light with longer wave length (Blue light has more energy than the red light per photon). Einstein of Red light (700 nm) has 80 K. cal. per mole-1.
Historical Account
Though Aristotle observed the effect of light on greening of the plants, it was Joseph Priestley who discovered that the plants are capable of purifying the air and called this process as dephlogistication. But Jan Ingen Houze demonstrated that plants can dephlogistication the air (by liberating O2) only in the presence of light. In dark, plants and animals behave similarly by releasing CO2 and phlogisticating the air (impurifying the air). However, it was Jean Senebier, who postulated that plants fix the atmospheric CO2 (fixed air) into organic compound and the oxygen that is liberated comes from CO2.
Plants – O2 (Priestley)
Light
Plants --- O2 (Jan Ingen Houze)
Light
Plants + CO2 --- Organic carbon + O2 (Jean Senebier)
At this juncture, Nicholas Theodore de Saussure postulated that H2O is used up in fixing the CO2 into organic compounds. This was an important step in understanding photosynthesis.
Light light
H2O + CO2 ------------- C + O2 C + H2O ---- (CH2O) n
plant plants
Robert Mayer, who was responsible for the theory, “that energy can neither be created nor destroyed”, clearly elucidated that the plants capture solar energy and store it in the form of chemical energy, in the form of chemical bonds.
Meanwhile, the importance of chloroplast and chlorophyll in fixing CO2, in the presence of light was made clear. But people still believed that the oxygen that is liberated during photosynthesis comes from the splitting of CO2 into C and O2. However, it was Robert Hill and Van Neil using radio active isotopes demonstrated that the oxygen that is liberated comes from the H2O and not from CO2 as it was believed earlier.
4H2O ----------- 2H2 + 2O2
Since then, numerous workers have contributed their part in understanding various aspects of photosynthesis. Blackman, Calvin, Arnon, Emerson, Park, Sanpeitro, Govindjee, Ellis, Parker, are the notables among the stalwarts who have made significant contributions in understanding the structure and functional aspects of Photosynthesis.
Photosynthetic Organelles: Chloroplasts
Plastids are the unique organelles found exclusively in plants (with the exception of fungi).

A. Grana, B. Plastid membrane, C. chloroplast-stromatic fluid, D. individual Granal circular structures-Thylakoid. E. outer surface membrane of chloroplast.
A fully developed chloroplast is bounded by a double membrane system within which, a fluid or a matrix called stroma is found. In this liquid 30-40 highly organized circular pack of discs called thylakoids organized into overlapping one on other and such groups are called Granum.

Within the membranous structures called Grana are found. Each Granum is made up 10-100 circular flat membranous discs called thylakoids. In these thylakoids the photosynthetic pigments and their associated proteins are organized into photosynthetic units. Refer page No.190 for the details of chloroplast structure.
Scanning transmission electron microscope
imaging of a chloroplast
(Top) 10-nm-thick STEM tomographic slice of a
lettuce chloroplast. Grana stacks are interconnected by unstacked stromal
thylakoids, called "stroma lamellae". Round inclusions associated
with the thylakoids are plastoglobulus. Scalebar = 200 nm. See (Bottom)
Large-scale 3D model generated from segmentation of tomographic reconstructions
by STEM. grana = yellow; stroma lamellae = green; plastoglobules = purple;
chloroplast envelope = blue. See.

If thylakoids are cut open by freeze fracture method and observed under an electron microscope, four surfaces can be made out as shown in the (fig-below). These surfaces are studded with numerous granular structures. Those structures found in the inner core of the membranes are called Quantasomes which act as photosynthetic units.
Photosynthetic units: Park and Branton discovered the presence of array crystalline particles, associated with the protein curried in thylakoid membranes. Such particles are called Quantasomes, which are nothing but photosynthetic units which are capable of absorbing solar energy as well as performing photo-chemical reactions. The larger particles are P.S-II and the smaller are P.S-I. Park and Sane have succeeded in isolating P-S-I and P.S-II by sonication and differential ultra centrifugation methods. This has led to the study of chemical analysis and function of each of these units.
Photosynthetic Unit I: It is smaller of the two photo-systems. The inter-granal lamellae or stromal lamellae have just P.S-I particles. But the granal thylakoid membranes are associated with P.S-II system. P.S-I is made up of 230-250 chlorophyll-a molecules. Along with the chlorophyll-b, Carotenoids, 25 K.D light harvesting proteins and unique molecule called chlorophyll-a 700, which exists in the form of a dimmer, are also present. This system is also associated with Fe-S containing Ferrodoxin (a reducing substance), a non-heme-iron protein called Ferredoxin (FRS), Cytochrome-b 6, and Plastocyanin and ATP dependent NADP reductase. In inter-granal lamellae cyclic photo-phosphorylation occurs, but in granal membranes the reduction of NADP to NADPH2 occurs. Recently Arnon came out with a view that P.S-I is totally absent in granal membranes but it is found only in stromal lamellae.
Photosynthetic Unit II: It is the larger photosynthetic unit, exclusively found or restricted to granal membranes. It is made up of a number of chlorophyll-a (more), chlorophyll-b, Carotenoids, 25 K.D. light harvesting proteins, specific Mn2* associated Z-protein complex (32 KD), Pheophytin, Plasto Quinone and Cytochrome-b 559. It also has a unique, Chlorophyll-a 680 molecule which exists in the form of a dimmer. Its major function is to liberate O2 and donate electrons to P.S-I system. However, Arnon feels that the originally believed P.S-I involved in the reduction of NADP is not P.S-I but it is another form of P.S-II system. Only time will tell the reality of its structure and function.
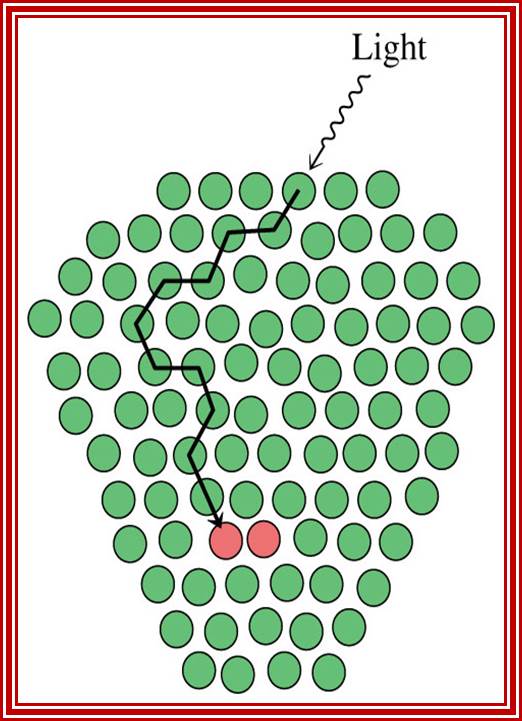
Transfer of energy: These two photo systems with their respective pigments and light harvesting proteins are oriented in the membrane in such a way; they can capture the fleeting photons randomly. All the molecules in each of these photo-synthetic units are spaced and oriented in such a way, captured photons, with specific amount of energy, are transferred from molecular cluster to the other by a phenomenon called inductive resonance. Here, the unit of energy that is transferred to is called exciton. The transfer of captured light energy is ultimately drawn to active centers.
Chlorophyll-a --- Chlorophyll-a 680
Carotenoids ---
Chlorophyll-b --- Chlorophyll-a 700
Photosynthetic pigments:
Pigment analysis of photosynthetic organelles, shows that chloroplasts contain green pigments (chlorophylls) and yellow pigments (carotinoids). Among chlorophylls, chlorophyll-a acts as the primary pigment and it is present in all photosynthetic organisms, with the exception of photosynthetic bacteria where the primary pigment is Bacteriochlorophyll. All other pigments act as secondary pigments.
Absorption Spectrum: The absorption spectrum of these pigments indicates that, chlorophyll-a shows maximum absorption and at 430 nm (blue light) and 662 nm (red light). Similarly, chlorophyll-b has absorption spectrum at 453 nm to 642 nm and carotinoids have an absorption band between 430 nm – 490 nm.
Energy carried by each photon of different wave lengths.
· What is the energy of a photon of blue light (λ = 450 nm) and of a photon of red light (λ = 700 nm) in units of eV = 1.6*10-19 J?
· Photons always move with the speed of light.
· Photons are electrically neutral.
· Photons have no mass, but they have energy E = hf = hc/λ. Here, h = 6.626*10-34 Js is a universal constant called Planck's constant. The energy of each photon is inversely proportional to the wavelength of the associated EM wave. The shorter the wavelength, the more energetic is the photon, the longer the wavelength, the less energetic is the photon.
Solution:
E = hc/λ.
Blue light: E = (6.626*10-34 Js)(3*108 m/s)/(450*10-9 m) = 4.4*10-19 J = 2.76
eV
Red light: E = (6.626*10-34 Js)(3*108 m/s)/(700*10-9 m) = 2.8*10-19 J = 1.8
eV
A 100-W incandescent light bulb uses 100 W (100 W = 100 J/s) of electrical power but only radiates about 15 W of actual visible light. Roughly how many visible photons per second hit the open pages of a typical hardcover book if the pages are about 2 m from the bulb and face it directly?
- Solution:
We have to make a variety of estimations and approximations to solve this problem.
To find the number of photons hitting the pages each second, we have to know the light energy hitting the pages per second and the energy per photon. We could compute the latter if we knew the wavelength of the light, but the visible light emitted by a normal incandescent bulb is a mix of wavelengths.
Let us estimate that the average wavelength of the visible light is about 550 nm, which is in the yellow region of the spectrum. This means that the average energy per photon is about
E = hc/λ = (6.626*10-34 Js)(3*108 m/s)/(550*10-9 m) = 3.61*10-19 J = 2.26 eV.
To find the number of photons hitting the pages of a book, we need to know the energy per second that falls on the pages. Let us assume that the light energy from the bulb travels uniformly in all directions. Imagine a sphere centered on the light bulb with a radius R = 2 m. Every second, 15 J or so of visible light energy crosses this sphere. If the light energy is spread uniformly over the sphere, then the intensity of the visible light or the energy per unit area per unit time in units of W/m2 at any point on the inner surface of the sphere will be
I = Plamp/(4πR2),
where Plamp is the power of the visible light emitted by the lamp (~15 W) and 4πR2 is the area of the inner surface of the sphere.
I = (15/(16π)) (W/m2)
One photon of blue light has 70 kcal energy and one photon of red light contains 40 kcal of energy.

Action spectrum and Enhancement effects:
Though individual pigments absorb light at specific wave lengths, not all the wave lengths are efficient in photosynthetic process either in terms of oxygen liberation or carbon fixation. If red light or blue light is provided alone, oxygen liberation would be inefficient on the other hand if both the lights are given simultaneously the photosynthesis. Though carotinoids absorb light at green and orange regions, instead of direct action, they transfer the energy to other pigments like chlorophyll-b and chlorophyll-a for effective function.
Mechanism of Photosynthesis
The process of light capturing and carbon dioxide fixation are separated by time and space. Structurally Grana performs the photochemical act by capturing, converting and conserving the solar energy in the form of chemical bonds in ATP and NADPH + H*. This is also involved in the liberation of O2. In this primary process, though certain steps are temperature dependent, others are light dependent and temperature independent reactions.
Stromatic fluid is the site for the utilization of light dependent granal products in carbon fixation. As this process is controlled by a number of enzymes, it is considered as a temperature dependent process. However, light induced photochemical reactions are extremely fast reactions are relatively slow and take place later as they depend upon the products of light reactions. The entire process can be simplified in the following equation and in the scheme.
Light Reactions
As the first phase of photosynthesis is light dependent, it is referred to as light reaction; however, this is also called. Hill’s reaction for, it was first demonstrated by Robert Hill.
Basic principles involved in photosynthesis is capturing of solar electromagnetic radiations at a particular wave length by pigment/protein complexes. The pigments act as antennas for capturing light rays in the form of photons in terms of quantums.

The light captured, with photons containing specific quantum of energy, is translocated among the atoms of pigments, where at a particular site an electron accepting the light energy jumps to a higher orbit. The orbit to which it jumps depends upon the amount of energy a captured photon provides. In the orbit the excited (higher energy electron) electron stays for a very short period of time, say -10^ 14 sec and then drops to down to the next lower orbit where it stays only for a very short time, then it falls back to its original orbit (ground state).

During downward fall the energy rich electron can be transferred to another compound. Thus, the light energy accepted in the form of a photon is transferred to another substance. This is a simple explanation of light harvesting process. If the energy rich electron is not used, the captured energy is released either in the form of fluorescence or heat.
Photoionization: To begin with, the absorption of photons of SEMR by pigment systems makes them excited.
Chlorophyll + Protein complex + light --- Chlorophyll -
(Ground state) - protein complex (CPC) (excited)

Overall view of photo-activated chemical Photo process;
The excited chlorophyll-protein complex reacts with water and splits the water into hydrogen ion (OH-n H+ions). This phenomenon is called photoionization.
Chlorophyll-protein complex (Excited) + H2O.
------- Chlorophyll – Protein complex + H* _ OH – (Ground state)
The photocopied products like H* + OH – ions are simultaneously accepted by the photosystem I and photosytem II respectively, found in granal membranes.
Production of reducing power: It is now certain that the synthesis of NADPH + H* is the co-ordinate function of P.S-I and P.S-II, where P.S-I is directly involved in the reduction of NADP.
When the light strikes, the photosystem I units, photons of the SEMR are trapped by a number of antennae like chlorophyll-protein complexes. This energy is transferred from molecule to molecule towards the special molecule called chlrophyll-a 700, which acts as the reaction centre or energy trap. When a Quantum of light energy is conveyed a chlorophyll-a 700, an electron at a particular site gets the energy and it is boosted to the next higher orbit or higher stage of energy.
In this process the energy that is responsible for boosting the electrons is now stored in NADPH + H*. This is a powerful reducing agent required for carbon fixation and it is released into the stromatic fluid as it is synthesized.
As the electrons from chlorophyll-a 700 are lost to NADP, the chlorophyll-700 molecules are rendered electrically positive. If electrons are not provided, these molecules become active and this condition is called photo-bleaching. However the electrons for chlorophyll a 700 come from P.S.II which is also moved in the act of electron transport.
Liberation of oxygen: Along with P.S.I, P.S.II units also capture photons and transfer the excitons to the active centre called chlorophyll-a 680 which exists in the form of a dimer. Similar to chlorophyll-a 700, chlorophyll-680 molecules also boost up the chlorophyll-a 700, chlorophyll-a 680 molecules also boost up the electrons to higher state of energy. As chlorophyll-a exists in dimmers, utilizing 2 Quanta of light, 2 accepted by photo system II, the larger photosynthetic unit of the two, has 32 K.D. Z protein complexed with manganese ions as active centre. With the loss of every electron from the terminal chlorophyll a 680 molecule, an electron is supplied by “Z” protein Mn2+ complex and thus this protein complex gets activated with every photon absorbed.
As a result, the positively charged Mn2*- Z protein complex readily reacts with negatively charged OH ions in water.
One more boosting of a pair of electrons are to be released from Z-Mn complex to receive two more OH – ions. When 4 such OH- ions are brought on to the two Mn-Z protein complexes, in such a way, the OH orbitals may overlap on each other. This may result in reorientation and reaction to produce 2H2O and a molecule of O2. This leaves Z – Mn2* complex free to receive another set of OH ions.
PS –II Z –Mn2* Complex + 4 OH –4 photons
PS II – Z Mn2* + 2H2O + O2
Non-Cyclic Photophosphorylation
The electrons that are boosted to higher state of energy from PS-II Chl a 680 – are immediately accepted by pheophytin.

Then the electrons are shuttled to a labile molecule called plastoquinones (PQ). The molecule simultaneously accepts 2H* ions and 2 electrons and gets reduced to PQH2, which in turn releases the H* protons into inner thylakoid space and simultaneously transfer the electrons to Cytochrome 559. This is accompanied with the loss of small amount of energy is released. This energy is utilized and stored in the terminal bond of ATP molecule. This phenomenon is referred to as non-cyclic photophosphorylation. From Cytochrome f, the electrons reach Chl a 700 through copper containing neutral and now they are ready for another cycle of electron boosting process. Meanwhile, as the electrons are lost from chlorophyll a 680 to chlorophyll a 700 the former molecule becomes positively charged. The electrons that neutralize Chl a 680 come from (OH) ions.
Cyclic photo phosphorylation: Photosynthetic unit-I that is found exclusively in inter granal lamellae is involved in a cyclic electron flow. During the down hill journey of electrons, energy is liberated and the same is utilized in the phosphorylation of ADP + Pi to ATP.

P.S-I absorb photons and energy is conveyed to the terminal Chl a 700 which exists in dimer form. The electrons are boosted to higher orbital, which is accepted by FRS. Then electrons are channeled through a series of electron accepters back to Chl a 700 molecule. When the electrons are transferred from FD to cytochrome b 6and form Cyt b 6 to Cytochrome 1, sufficient amount of energy is released. The energy that is released during this shuttle is due to the difference in the Red-O potential between the oxidant and reductant. This energy is captured and utilized in the formation of a high energy terminal bond of ATP molecule.

Light reaction and its products:
During light reaction solar energy is absorbed by chlorophyll light harnessing complex and utilize water for photochemical reaction to produce 3 ATP, 1 NADPH + H*, 2H2O, 1 O2 per cycle of reactions.

Particularly ATP and NADPH + H* are released into stromatic fluid. Oxygen either escapes or it is utilized by respiratory process.
Dark reaction or Calvin Cycle;
Stromatic fluid consists of all the enzymes required for carbon dioxide fixation. Calvin et al, have elucidated all the biochemical steps involved in this process. Calvin is probably the one and the only biochemist, who has been awarded the Nobel Prize for this kind of work.

This pathway is also called C 3 pathway, for the first stable intermediate product produced is a 3 Carbon organic acid; further it is these 3 carbon components that play an important role in the synthesis of Glucose. This process takes place in 3 steps: 1) Carbon oxidation 2) Reduction 3) Regeneration.

Carbon Fixation
Ribulose monophosphate (5C) an intermediate component of carbohydrate metabolism first gets phosphorylated by an enzyme Ribulose kinase and a molecule of Ribulose di phosphate is synthesized.
RUMP + ATP – RUDP + ADP. 1
The RUDP (5C) acts as an acceptor of CO2 found dissolved in the stromatic fluid. RUDP carboxylase is responsible for the addition of CO2 to RUDP, when a highly stable 6C compound, formed, break up into two 3 Carbon compounds called 3-Phosphoglyceric acid (PGA).
RUDP + CO2 – 2 PGA 2
(5C) (1C) (3C)

Reduction
Before the 3-PGA gets reduced, the 3-PGA is primed up by phosphorylation by phosphoglycerokinase to produce. Di phosphoglycerate (Di PGA). A molecule of ATP is consumed in the process.
3-PGA + ATP = 1, 3 – DiPGA + ADP
(3C) (3C)
The DiPGA gets reduced by NADPH + 1H*to
Phospho glyceraldehydes (2 PGALD). The enzyme involved is glyceraldehydes dehydrogenase.
DiPGA + NADPH + H = PGALD + NADP + Pi
(3C) (3C)
For the fixation of one molecule of CO2 into 2 molecules of PGALD, one molecule of RUMP, 3 molecules of ATP and 2 molecules of NADPH + H* are required. The sum of Carboxylation and reduction process to produce one molecule of Glucose has been given in the following equation.
6 CO2 + 6 RMP + 18 ATP + 12 NADPH + H*
= 12 PGALD + NADP + 18 ADP + 12 Pi

Regeneration
In this process, 12 PGALD are subjected to a regeneration process in which 6 RUMPs initially consumed are regenerated. At the same time one molecule of glucose is produced.
Out of 12 PGALD, five are converted into Dihydroxy Acetone Phosphate (DiHAP) by an isomerase enzyme.
5 PGALD = 5 Di HAP 6
In the next step, 3 molecules of PGALD combine with 3 molecules of Di HAP to form 3 molecules of Fructose-1, 6-Di Phosphate. The enzyme is Aldolase.
3 PGALD + 3 Di HAP --- 3 Fructose-1, 6-Di P
(3c) (3c) (6c)
These Fructose-1, 6 di phosphates are subjected to phosphate action in which one phosphate is removed to produce Fructose-6 phosphates.
3 Fructose-1, 6-diphosphate --- 3 Fructose-6-phosphate.
Out of the 3 Fructose 6-phosphates, one is converted into Glucose 6-phosphate by phosphogluco isomerase.
1 Fructose 6-p ------ I Glucose 6-p
(6c) (6c)
The remaining 2 Fructose-6 phosphates combine with 2 molecules of phosphoglyceraldehyde, to undergo a rearrangement process in which 2 molecules of Xylulose phosphates are produced. The enzyme is transketolase.
2 Fructose6-p + 2 PGALD
(6c) (6c)
= 2 Erythorose-4-p + 2 Xylulose-5-p
Then 2 Erythrose 4-P combine with 2 Dihydroxy acetone-P by another Transketolase enzyme to form a carbon Sedoheptulose-1, 7-diphosphate, which immediately undergoes phosphotase action to produce sedopheptulose7-phosphate.
2 Erythrose-P + 2 Di HAP
(4c) (3c)
= 2 Sedoheptulose-1, 7-di P
2 Sedoheptulose-1, 7-di P- 2 Sedoheptulose-7-p
The two sedoheptulose 7-phosphates thus produced combine with the rest of 2 molecules of phosphoglyceraldehyde to produce 2 molecules of Ribose-5-phosphate and 2 molecules of xylulose 5-phosphate. The enzyme involved is Aldolase.
2) Sedoheptulose-7 – P + 2 PGALD
(7c) (3c)
= 2 Ribose-5 – P + 2 xylulose-5 – P
(5c) (5c)
The two ribose-5 – phosphates and four xylulose-5 – phosphates (reaction 9 &12) are converted to Ribulose monophosphate by the isomerase and epimerase respectively.
2 Ribose – 5 – P = 2 Ribulose 5 – P
(5c) (5c)
4 Xylulose 5 – P = 4 Ribulose 5 – P
(5c) (5c)
The RUMPs thus produced are again used up in another cycle of CO2 fixation. The results of the pathway can be summed up as.
7 PGALD + 5 Di HAP = 1 Glucose + 6 RUMP
Hetch & Slack pathway or C4 pathway

Though Calvin cycle or C3 pathway is prevalent in the majority of Dicot plants some tropical Graminae or Grasses operate carbon fixation by another pathway called Hatch and Slack pathway. (This is named after the discoverers). This is also called C4 pathway because the first intermediate compound synthesized, during carbon fixation is a four-carbon organic acid. This method is considered as an efficient method of carbon fixation. The uniqueness of this process is that C4 pathway and C3 pathway operate in the same plant, but in different structures. In Graminae members like Sorghum and Sugarcane the vascular bundles found in the vines are surrounded by a single layer of bundles heath cells, which in turn are surrounded by mesophyll cells. Chloroplasts found in mesophyll cells and bundle cells show different structure as well as functions.
Factors that control photosynthesis:
Photosynthesis being a photo biochemical process, it is controlled by various factors to achieve maximum yield. Under optimal conditions if any one of the factors is deficient the rate of photosynthesis suffers. If such a factor is increased the rate also increases. Thus, the rate under controlled conditions shows minimal or optimal or maximal quantities. For example, when photosynthesis is operating under optimal condition, if carbon dioxide is deficient, the rate of reaction increases, but reaches saturation point at which condition, another factor, say light, may act as a limiting factor. If the intensity of light is increased, again the rate increases to maximum and reaches saturation point. This may be further increased on increasing the concentration of enzymes. Thus in living systems various factors and their concentration readily affect the efficiency of photosynthesis.
Pigments: Green chloroplasts contain green as well as yellow pigments. Among green pigments, Chl-a & Chl-b are invariable present; among yellow pigments carotenes and xanthophylls are present; chlorophyll-a, particularly Chl-a 700 and Chl-a 680 are directly involved in the primary process of photochemical reactions. All other pigments act as secondary pigments. Even though Chl-a 700 and Chl-a 680 act as active centers, other pigments absorb light at specific wave lengths and they are conveyed from molecule to molecule towards active centers. Here, the energy is transferred by inductive resonance in the form of excitons.
These pigments get organized in the thylakoid membranes in the form of photosynthetic units called P.S-I and P.S-II. Both P.S-I and P.S-II are found in thylakoid membranes, but P.S-I is exclusively found in the inter granal lamellae. In thylakoid membranes, P.S I and P.S-II are uniquely oriented towards each other, in such a way, their function is co-coordinated and they are responsible for oxygen liberation (P.S-II) and NADP reduction to NADPh2 (P.S-I). During the electron flow from P.S-I, the light energy is converted to chemical bond energy (ATP) by Non-Cyclic photo-phosphorylation.
Generally, at high intensity of light, chlorophyll molecules get bleached by photo-oxidation, but carotinoids that are present, protect the chlorophyll molecules from photo-oxidation. If there is any deficiency in any of the pigments and required factors for their organization, it affects the process drastically; sometimes the effect may be lethal too.
Light:
In photosynthesis light provides energy. At the same time it stimulates the entire process. Basically this process is controlled by the quality and the quantity of light that is received.
The solar electro-magnetic radiation consists of a wide spectrum of individual rays, which differ in their wave lengths. These radiations travel in the form of discrete energy packets called photons. The amount of energy in each photon is called Quantum, which mainly depends upon the wave length of the radiation. Shorter the wave length, greater is the energy of longer the wave length lesser is the energy of the photon. For example, the red light of the visible spectrum (white light) has longer wave length and lesser energy when compared to the shorter wave length of the blue light which has higher amount of energy.
This energy relationship between photon and wave length is shown in the figure 87.
Quantity of light
Chlorophyll a, Chl.B B-carotenes and Xanthophylls absorb different wave lengths of visible light (Cal a, chl. B, B-carotene & xanthophylls). This is called the absorption spectrum. Even though these pigments absorb light at shorter wave lengths, the photons are transferred to the active centers which have an absorption spectrum at longer wave lengths.
Chlorophyll molecules, because of heterogeneity in their structure absorb white light at Blue as well as Red wave lengths. If blue or red light is given singly photosynthesis will be never efficient; on the other hand, if both the lights are given simultaneously, the process becomes efficient in releasing O2. This effect is called Emerson’s enhancement effect. That means photosynthesis requires both red and blue lights for its efficient function.
Quantity and intensity of lights: The intensity of the light depends upon the distance of the source, and it is measured in terms of foot-candles. It has been found that at low intensity, photosynthesis is at its minimum but with the increase in intensity the rate increases and reaches saturation at about 2000 to 2500 foot candles. At higher intensities, however, pigments get bleached and the process suffers. The inhibition of photosynthesis by photo-oxidation at higher intensities is called ‘Solarization’.
Quantum requirement / efficiency
Light is measured either in terms of quanta or intensity of light. The efficiency of light is measured in terms of number quanta required for either the liberation of a molecule of O2 or for the fixation of a molecule CO2 into Glucose. Quantitative measurement of red light required for the liberation of one molecule of O2 is found to be about 4 quanta and another four are required for the production of two molecules of NADPH*. During this process 2 molecules of ATPs are also synthesized; totally 8 quanta are required.
In Cylic photophosphorylation 2 molecules of ATP are synthesized per 2 quanta of light. 2 ATP cyclic 2 quanta.
6 molecules of CO2, 18 molecules of ATP and 12 molecules of NADPH2 are required. This is approximately equivalent to 60 quanta.
6 CO2 + 6 RUMP + 18 ATP + 12 NADPH + H ---------- C6 H12 O6 + 12 NADP + 18 ADP + 18 Pi.
But the amount of total energy that is actually stored in a molecule of Glucose is about 696 or 700 K. cal. Thus the efficiency or photosynthesis in terms of energy required and stored, gives the value of 60%. Probably the other 70% of the energy that is not fixed may be released in the form of heat which may be utilized for enzymatic and other cellular activities.
Carbon dioxide (CO2)
It has been estimated that about 300 million years ago the percentage of CO2 in the atmosphere was about 20%. Today, with burning of all kinds of fuels (including biological oxidation) the concentration of CO2 in the atmosphere is just 0.03%. Naturally one will be tempted to ask “What has happened to the rest of the CO2?” Nature has fixed this CO2 in the form of organic fuels by photosynthetic process and the same is stored as the organic fuel in the inner depth of earth. Nevertheless, plants growing on land and in ocean fix about 146 billion tons of carbon annually which is approximately equivalent to the fixation of 13x 1012 K.cals. of solar energy.
Generally, in a vast landscape full of green plants, CO2 becomes deficient by about mid-day, and photosynthesis and its yield suffers. By increasing the concentrate of CO2, the rate of photosynthesis increases, so also its yield. Different plants have different optima for CO2. C4 plants are considered as highly efficient plants than C2 plants with respect to carbon fixation.
Water: Water being sole source for electrons and protons, its supply is very important in photosynthesis. But under water deficit conditions, the rate of photosynthesis falls drastically, if higher concentration of H2O does not show any enhancing effect.
Temperature: Photosynthesis, which is a photo-chemical reaction, requires enzymes for its various biochemical activities temperature has a significant effect on controlling the rate of photosynthesis. Different plants have different optimal temperature. Desert plants have higher optimal temperature of 35-400 C. On the other hand, other mesophytic and water plants function efficiently at 25-300C.
Internal and Genetic factors
Even under optimal environmental conditions, without the proper cellular machinery, no metabolic activity can function efficiently. The synthesis of various cellular factors ultimately depends upon the genetic potential and its expression. Plants with superior genetic potential can function very efficiently when compared to inferior ones. All enzymes, pigments, light harvesting proteins, co-enzymes, and their synthesis things is of utmost importance in the proper functioning of photosynthesis which ultimately controls the photo-synthetic yield.