PLANT GROWTH AND DEVELOPMENT.
A big banyan tree grows out of a tiny seed. A microscopic leaf initial in the special bud of Victoria regia develops into a large leaf on which a man can sleep. Like that, many plants start this development as tiny structures and grow to huge sizes. Whether they grow to be giant plants or not, all plants start their development as unicellular zygotes and by repeated cell divisions and differentiation they grow into fully developed plants and live, some in short time some take many months and years.
The development of a plant is a highly complex phenomenon. A zygote, with the memory of growth, in the embryo sac utilizing available nutrients, starts dividing mitotically into two, and then four and so on. Among them specific cells, have specific genes are expressed and undergo cellular orientations, which determines the future developmental pattern in which a group of cells develop into stem and the other group into root system. This type of polarity is determined and fixed at a stage as early as 4-8 celled embryo. Even at this stage, all cells look alike, but as specific genes have expressed and the predetermined cells undergo cell division and one finds that some of the cells remain exclusively for cell divisions and the other cell-derivatives develop into different cell types which in turn develop into different but specific structure of different plant organs. Cells derived from such a single or a group of mother cells either divide further and increase their number and then differentiate or directly differentiate and develop into new cell types. The whole process is derived from specific genes expression induced by specific factors. Early genes expression is predetermined, this leads to differential cell types endowed with specific functional characters and further develop into specific types of cells with specific functions. Which genes are expressed and how is a mystery; only molecular studies reveal, how and why?.
Inspite of the complexity of these developments, it is clear that the growth involves cell division (numerical increase), cell growth in 3-D increase in size and differential expression specific genes leads to differentiation (3-D increase in size) of cells into specific cell types. The most complex problems are what make the cell to divide in a particular way and differentiate? What makes the cells to grow? How cells develop into specific cell types? Above all, ‘What are the molecular events that determine the course of their differentiation and development’? Answer to the above questions is not easy to find. Molecular aspects of growth and differentiation, an exciting field of study. Only future work can throw light upon this developmental process.
Types of Growth: Growth is of two types; 1) Indefinite or unlimited growth exhibited by root, stem and their branches, 2) Definite or limited growth exhibited by leaves, flowers, fruits, etc.
Rate of Growth: Under given normal conditions different plants show different rates of growth. This is due to their genetic potentialities. For example, Bamboos vulgaris (Bamboo) shows a very high rate of growth, about 45 cm / day. On the other hand, Cycas, a Gymnosperm, plants grow so slowly that it takes 100 years to reach a height of 10 ft or so.
When different phases of growth are compared with the different structures involved in growth, a definite correlation can be made out. If the radicle of a germinating seed is taken for the study, the extreme tip of radicle is made up of a group of actively dividing cells, called region of meristem and the phase of growth in this region is called Formative phase. Just beyond this meristematic region, the cells which are derivatives of meristematic cells, show active metabolism and are in a rapid process of cell elongation called the region of elongation which corresponds well with the phase of elongation or enlargement. Just beyond this region, the cells are engaged in the process of differentiation where different cells are undergoing differential gene expression which leads to modifications into different cell types. This region is called the region of differentiation and maturation, a remarkable feature.
Growth curve: In graphical studies on growth, where increase in length of different plants is plotted against function of time, a sigmoid or “S”-shaped curve is obtained. This is generally referred to as growth curve. The analysis of this curve shows three distinct regions, indicating a lag phase, a log phase and a steady phase, which very well correspond to the three regions, called phases of growth. The middle region of the curve shows the grand period or exponential period of growth where plants exhibit maximum growth rate.
Growth in terms of functions is the summation of many gene expression and complex metabolic and cellular activities. Basis on the intrinsic and extrinsic features, growth can be defined as an irreversible increase in protoplasmic mass and dimension with time. Though many factors like nutrient supply, oxygen, temperature, light and others have their own effect on growth and the regulating substances particularly phytohormones play a significant role in growth and development of plants.
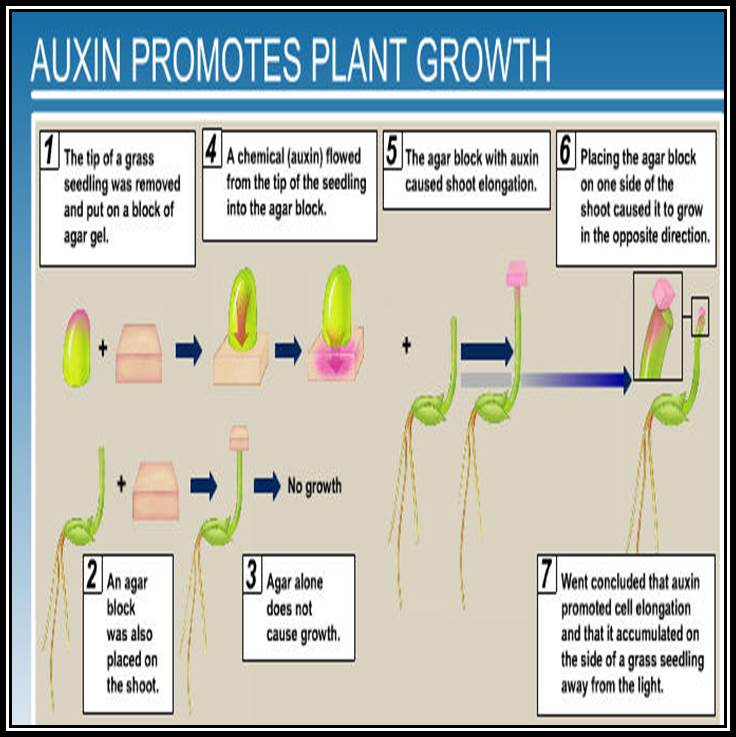
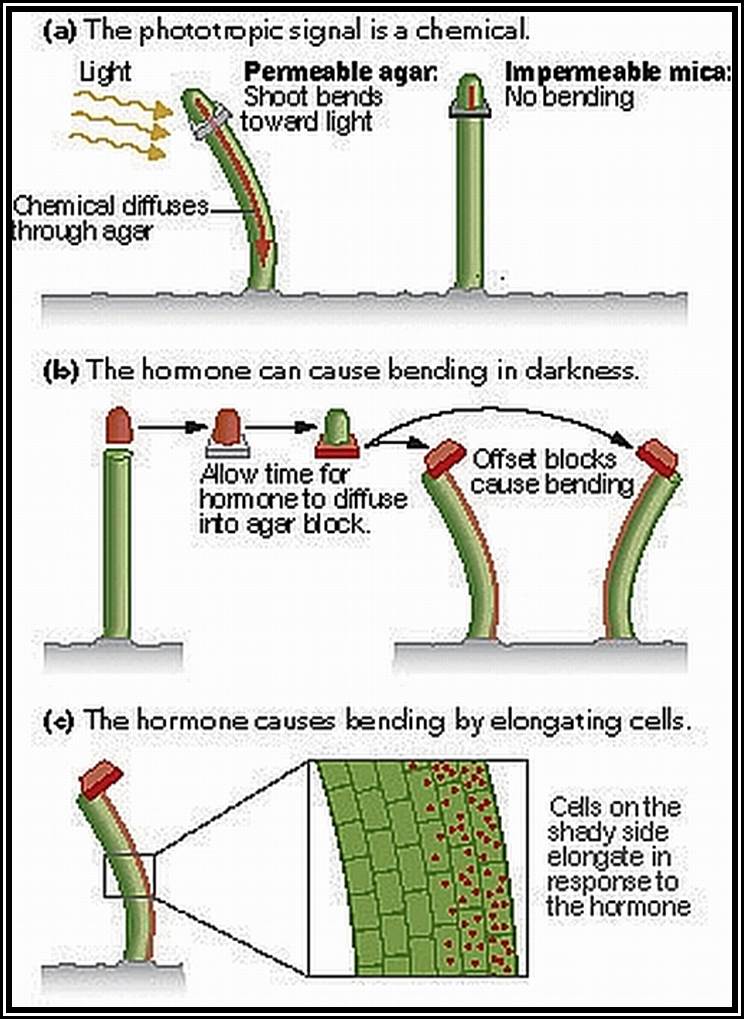
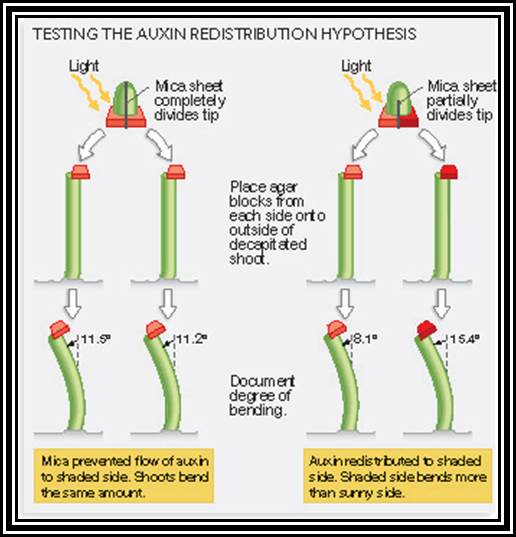
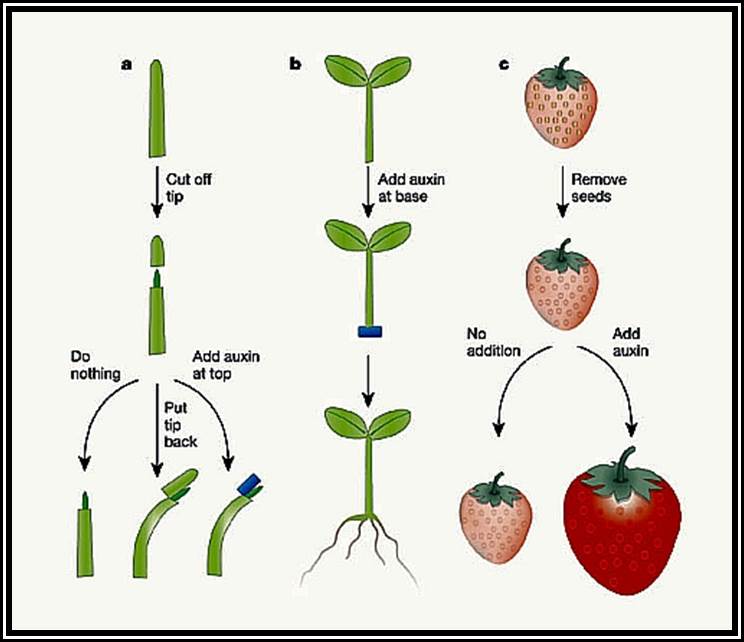
Discovery of growth hormones: The discovery of growth hormones dates back to the days of Charles Darwin who described the plant curvature as a response to the light in the book called “The power of movement in plants”. When the stem tip of the canary grass is exposed to unilateral light, the coleoptiles tip bends or curve towards the source of light. If such stem tips are covered with metal cap, so as to exclude the light falling on the tip, they remained insensitive and don’t show any curvature.
It took a long time to establish that the growth curvature is due to some substance synthesized in plants. Boysen Jensen demonstrated the existence of such materials in stem tips by simple experiments. Paal, on the other hand confirmed the presence of growth promoting substance in the stem tip. In his experiments, Paal decapitated the coleoptiles tip of the grass and placed a block of gelatin and then replaced the cut tip on the gelatin block and exposed it to unilateral light. In response to light, the tip, showed bending movement, thus the demonstrated that some substance found in the tip has moved downwards through the gelatin and brought about the growth.
However, F.W. Went has gone a step further and collected this unknown substance on Agar blocks by placing the cut coleoptile tips on them. By placing such loaded agar blocks on the decapitated stems, he demonstrated trophic curvature and in fact he established a method called bioassay by means of which he established the relationship between the concentration of the growth substance and growth response.
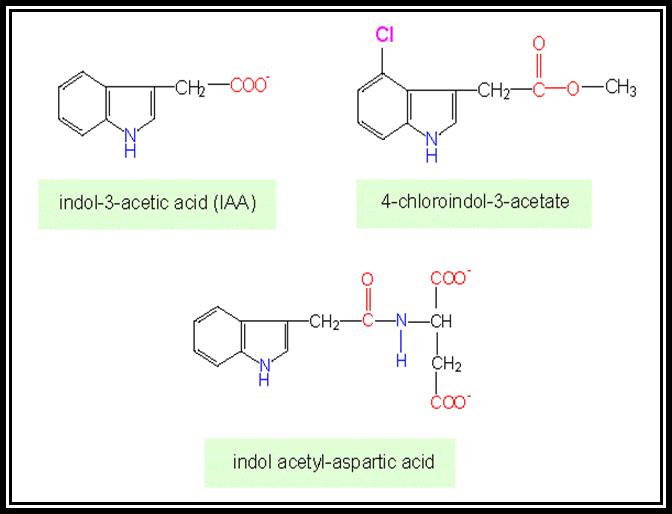
It was left to Kogal and Haugen Smit to isolate and purify this growth promoting substance, by charcoal adsorption method and called it as Heteroauxin. Its chemical nature was established as Indole 3-Acetic Acid. This was not a new substance for them, for it was already discovered by Salakavaski nearly fifty years ago. But its biological activity was not known at that time.
Indole 3-Acetic Acid (IAA)
Since the discovery of IAA (a natural auxin), many synthetic auxins have been developed and they were in the use in horticulture and agriculture, while the studies on Auxin and its effects were going on.
Auxin
Auxin, comically, Indole-Acetic acid (IAA) and it is a natural and a basic phytohormone found in almost all plants. It is synthesized in the extreme tips of the stem and roots at a region just above where procambial cells are undergoing differentiation. Then this substance is transported towards the respective basal regions where they bring about their effect on growth differentiation. Since the discovery of IAA as a natural auxin, various synthetic auxins have been identified and synthesized. Ex. Indole 3-Butyric acid (IBA), Indole propionic acid (IPA), Naphthalene Acetic Acid (NAA), 2-4 Dichloro phenoxy Acetic acid (2-4-D) etc. Such indoles are fund in our food items called HINGU (kannada). These auxins are being in use in horticulture and farming industry.
Site and synthesis: Auxins are generally synthesized in the apex of roots and shoots and in very young leaves. Even pollen tubes and fungal mycelia also produce IAA. Tryptophan, an amino acid, is the precursor for IAA. By decarboxylation and deamination process Tryptophan is converted to IAA (Indole Acetic Acid).
Distribution and transport of Auxin
If a seedling is taken and cut into equal segments, then Auxin found in such segments and estimated, it has been observed that highest concentration of auxin is found in the stem tip and the next highest is at the root tip, but its concentration is nowhere near the concentration found in stem tips. The concentration gradually decreases towards their respective bases and the minimum is found at cotyledon region.
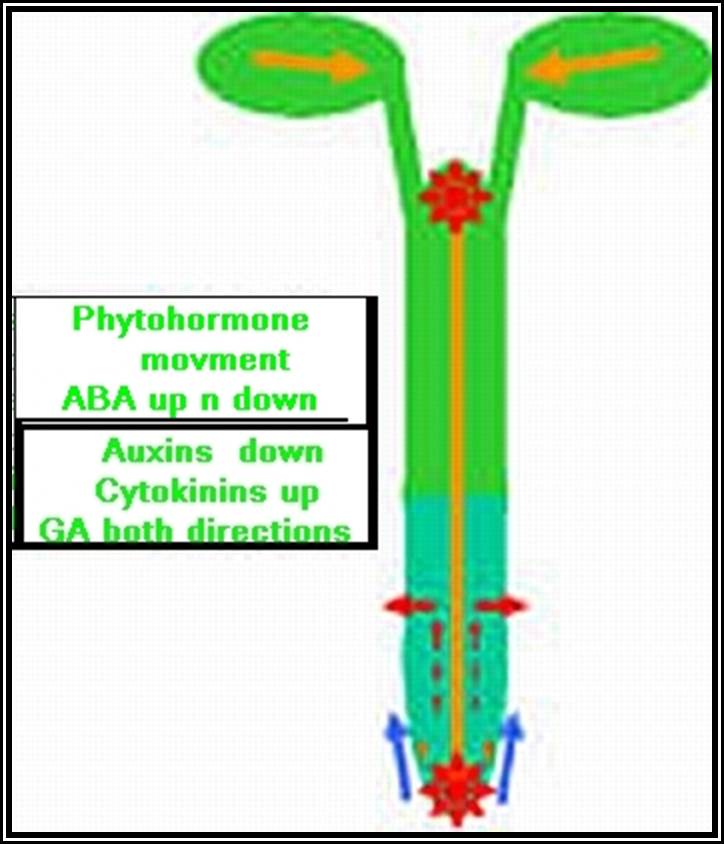
Auxin has been found to exist in two states. One is in bound form and other is in the free form. The bound form is the auxin which is associated with auxin binding / mediator proteins. Furthermore, it has been found that the bound form is active. The equilibrium of these two states actually controls the growth and differentiation.
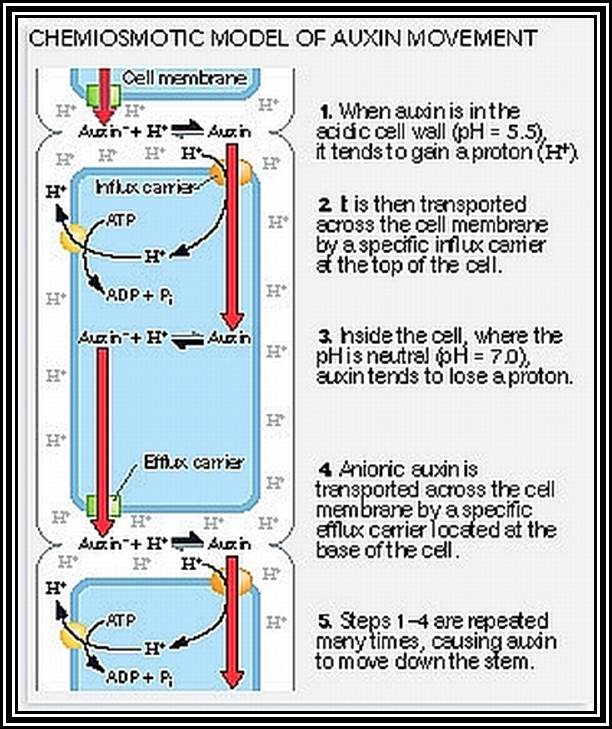
Transport: Auxin that is synthesized at the tips is transported towards their respective bases. This type of movement is called basipetal movement. However, auxins are also found to move in acropetal direction i.e., from base to tip, but the ratio of basipetal to acropetal is 1:3. The basipetal movement is generally along the concentration gradient, i.e., from high concentration to low concentration, and it is probably through simple diffusion method. On the contrary the acropetal movement has been found to be energy dependent and hence it is an active process.
The pathway of auxin transport in the stem or root has been found to be the sieve tubes of phloem elements, which are actually responsible for the transport of its food substrates. The rate of movement ranges from 6-4 mm/ hr to 20 mm / hr. This rate is many times higher than the rate of simple diffusion. Hence it has been discovered that the mechanism of transport is partly by diffusion and partly by active process.
Physiological effects of Auxins:
Auxins play a very important role in growth and development of the plant. It is needless to mention here, that the total development of plant body is not just because of one hormone, but with many other hormones, it is the balanced interaction between them that causes the wholesome growth of the plants. However here we will restrict our studies to only auxins and its effect on general metabolism, cell elongation, phototropism, apical dominance, root initiation, parthenocarpy and abscission.

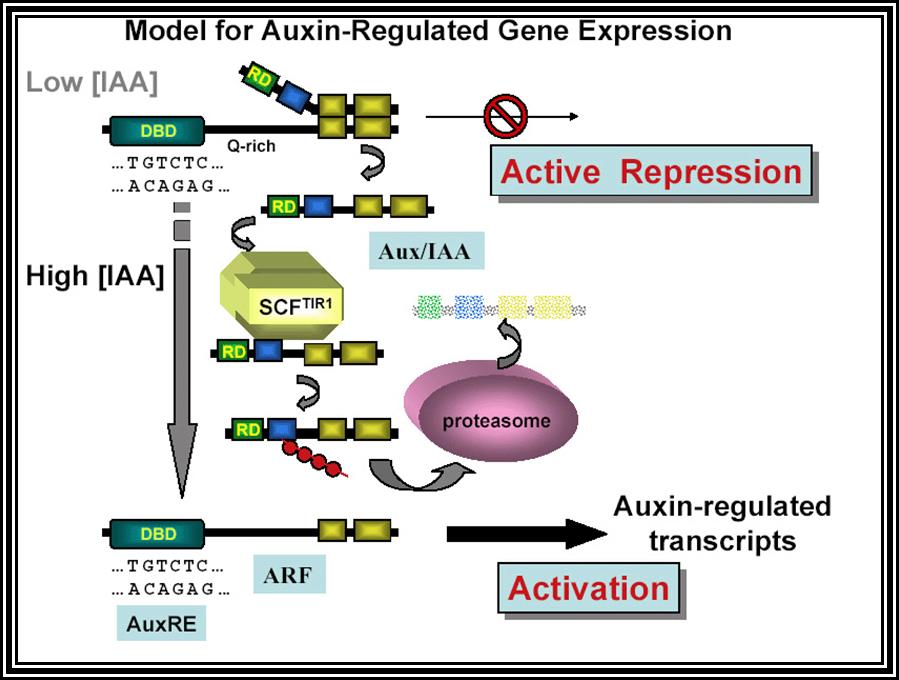
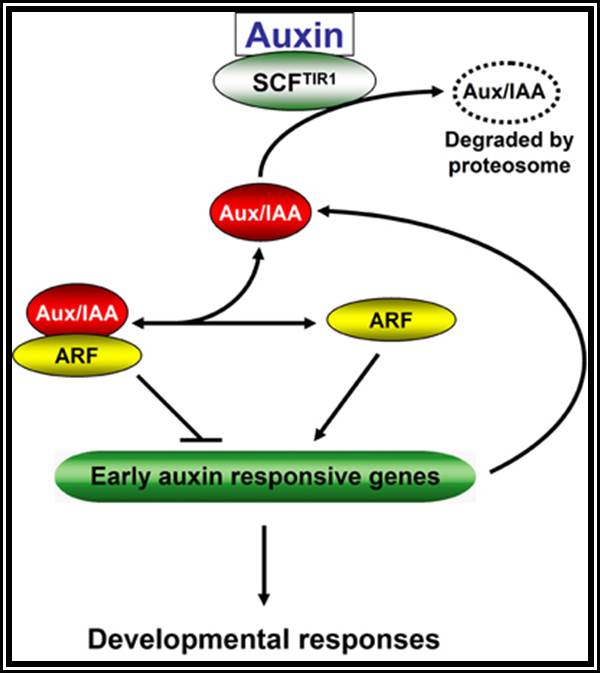
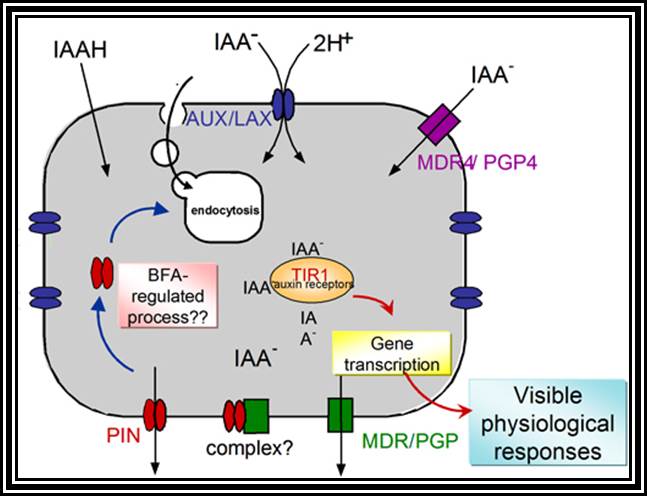
All its physiological effects start with its signaling pathways. The diagram just shows a simple module of auxin signaling pathway.

General Metabolism
Auxin is involved in the general metabolism in various ways and its degree of effect is concentration depend. If the plant body, or excised segment of the plant body
is exogenously supplied with auxins (natural or synthetic), various biochemical reactions show increase rate of activity. Auxin’s effect on respiration is almost immediate; various enzymes of it gets activated. The stored food substances are drawn out and degraded to simpler components which are utilized in biological oxidative steps resulting in the increased synthesis of ATP, stimulation of the translation system which is responsible for protein synthesis. This early increase in protein synthesis, is independent of RNA synthesis for, the increase in RNA synthesis is as late as 30-45 minutes after auxin application; hence it is deduced that the early increase in protein synthesis is due to activation of pre-existing mRNA protein complex or it may also be due to activation of protein synthesizing mechanisms which draws RNAs from the pre-existing pool.
Auxin activates RNA synthesis little later through certain unknown mechanisms; it causes a differential gene activiation. As a result, ribosomal RNA, t.RNA and specific m. RNAs are synthesized at an increased rate, which provides a pool of them for the further increase in protein synthesis. Thus, it has an overall effect on the synthesis of various cellular components and this increased metabolism has a profound effect on the growth of cells in particular and plants in general.
Auxin when it is transported into the cell, it binds to auxin response factors (ARF), which in turn binds to Aux -protein complex. This complex, positions on auxin response gene promoters and repress their gene expression. When ARF-auxin binds to Aux/IAA proteins, the repressor proteins are fed to proteosomes through SCF complex. This makes the auxin responsive gene promoters free from repression and become active. This just general mode of gene expression by Auxins.

Cell expansion
Within 5-10 minutes of application of auxins to isolated epicotyls, or intact tip of the plant or excised segments, growth in their length starts. This early effect is rapid and spontaneous. This rapid growth continues up to 30-45 minutes and then slows down but remains steady. This is also accompanied with an increase in cellular respiration, protein synthesis and the rapid extrusion of protons, in particular Hydrogen ions through the cell walls.
Another significant observation of this early rapid growth is insensitive to the inhibition of transcription (RNA synthesis) and translation (protein synthesis) – ex. Actinomycin-D at high concentration inhibits RNA synthesis completely and cycloheximide (CHI) inhibits cytosol protein synthesis. This clearly suggests that the early rapid growth requires neither the synthesis of RNA nor the synthesis of proteins. But this early effect of auxin is totally inhibited by colchicines at 0.5 to 1% concentration. Colchicine, an alkaloid from the tubers of Colchicum autumnale brings about the inhibition of tubulin (TB) polymerization into long microtubules, which act as the cytoskeleton for mechanical support in the cytoplasm. The colchicines effect on early growth further indicates the important role played by the assembly of microtubules in providing a mechanical structure and the force for all expansion.
Increased metabolic activity, particularly the degradation of osmotically inactive starch etc. leads to the increase of osmotic pressure (they are related to each other) and a steep DPD gradient is created. This facilitates the inward diffusion of water; consequently, turgor pressure builds up within the cells. This force acts on all sides of the cell wall and forces the cell to expand in length and breadth. However, recent investigations have shown that the cell elongation can takes place under negative pressure also.
The above features suggest that the process or the mechanism of cell elongation is complex and multiphased, but highly regulated. Various theories have been proposed from time to time and none of them could prove their claim because of one or the other drawbacks. Here we will restrict to a comprehensive account of cell elongation.
Early phase: The auxin’s effect, in the early phase on cell growth, is rapid. This is insensitive to the inhibitors of transcription and translation and also insensitive to colchicine and respiratory inhibitors like cyanide, DNP etc. From this, it can be deduced that cell elongation does not require new RNA and protein synthesis, but it requires ATP and ATP dependent tubulin polymerization into microtubular cytoskeleton. Auxin at the early stages activates and increases the rate of respiratory metabolism. With the result ATP pool builds up rapidly. Auxin also activities membrane bound nucleating centre of tubulin polymerization. These nucleating centers, utilizing ATP and tubulin monomers found in the cytoplasm, start polymerizing and soon build up long microtubules. These, in turn, start orienting parallel to the long axis of the cell, which probably requires cytochalasin-B sensitive microfilaments. With the rapid increase in microtubules, oriented in longitudinal plane, considerable force is exerted on the cell boundaries.

As the microtubular polymerization is building up, ATP ase / H* gets
activated, and H* ions are pumped out into the interspaces of cellulose
fibrils; acidic pH in turn activates cellulases enzymes, which starts loosening
up the cell-wall cellulose fibres. This loosening up of cell wall concomitant
with acting as a mechanical force-the cell expands in length. This process
starts 4-5 minutes after application of auxins and the elongation continues
rapidly for about 20-30 minutes.

The late phase starts around 30-40 minutes after auxin application. This is slower than the first phase, but growth is steady. This phase is sensitive to Actinomycin-D, CHI, Colchicine and respiratory inhibitors, indicting that this process requires metabolic energy, tubulin-polymerization, RNA synthesis as well as protein synthesis. As tubulin pool gets exhausted in the first phase, it has to be replaced with more protein synthesis.

Apart from the said proteins, the cytoplasm requires the synthesis of other proteins for the increase in the mass of protoplasm. It is quite possible that the early activation of early translation may have significant effect on gene activation. Thus, it takes some time for the second phase of growth to set in. The second phase provides all materials for sustained growth.
Growth Movements: The phenomenon of bending movement of plants in response to light stimulus is called Phototropism, the growth of roots towards soil is called Geotropism. The curvature growth due to the touch is named as Thigmotropism etc. Such differential growth movements are due to differential concentration of auxin. The stem tips and root tips have different optima of their auxin requirement to show maximum growth. The mechanism of these movements will be explained in the chapter on growth movements.
Auxin brings about phototropic movement because differential accumulation of auxin at the exposed regions.

Root initiation: Vegetative propagation of stem cuttings is a normal practice in horticulture, floriculture and agriculture. Out of some stem cuttings which are put into soil, a few produce roots easily but others may or not produce roots. The roots that develop from any part of the plant body other than the radicle are called adventitious roots. The development of such roots from the stem or any other part is a phenomenon of dedifferentiation or redifferentiation.


Stem is quite different from the root. Still, some of the tissues, found in the stem undergo dedifferentiation and develop into roots. This dedifferentiation and growth of roots from the stem is initiated by auxins Indole Acetic Acid, IBA, NAA, and other hormones. When applied to stem cuttings they induce the formation of roots. IBA has been found to be very good in this regard.
The mechanism of IBA induced root formation is very complex and poorly understood. However, it has been

shown that IBA at the very early stages of application to stem cuttings stimulates translation system (Protein synthesis) without the concomitant increase in the synthesis of m. RNAs. This indicates that the pre-existing m.RNA-protein complexes are drawn towards protein synthesis: it shows increased rate of protein synthesis. Some of the proteins thus synthesized in turn activate differential nuclear gene activation. As a result of it, a cascade of events occurs. Generally, there is an increase in the synthesis of all cellular proteins. At the same time there is an enhanced rate of synthesis of some specific proteins. Also, some new proteins are synthesized. Among these, tubulin, a membrane bound fraction, is synthesized at a high rate. It is believed that this protein along with microfilaments gets assembled into microtubules which orient themselves in a specific way so as to facilitate the polarity fixation for cell division.
In response to auxins, some of the cells in the pericycle region get stimulated and the required macromolecules are synthesized by differential gene activation. Thus, these cells endowed with these new potentialities divide and redivide, and soon they organize into root primordial. They in turn develop into new roots which penetrate through cortical cells. The time taken for this new root formation, in response to auxin supply is just 24-36 hours. This effect of auxin induced new root formation has been well exploited by horticulturists and others.
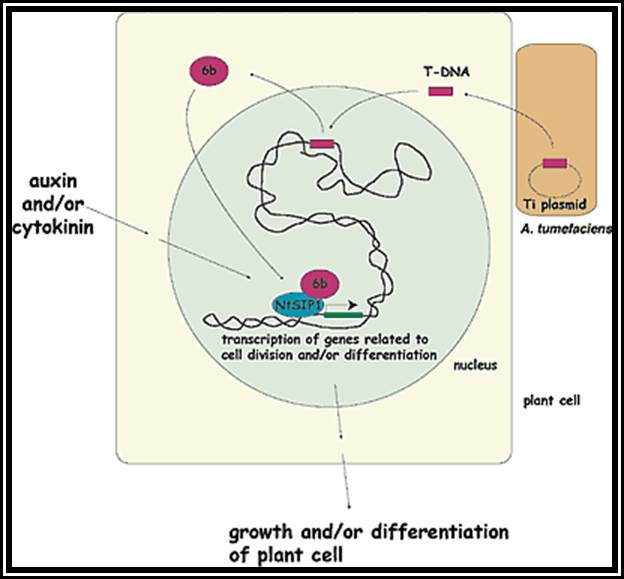
The following diagram shows how auxins interact with cytokinins in eliciting growth responses.

Apical dominance: The active growth of terminal bud inhibits the development of lower axillary bunds. This effect is called apical dominance. Many plants like conifers and other exhibit the apical dominance so predominantly that the plant grows tall with few branches and the plant appear conical in shape. But plants like banyan, mango, tamarind do not show that much of apical dominance, hence these plants spread outwardly. This phenomenon of non-apical dominance has been attributed to the differential concentration of auxin and nutrient supply to the axillary buds.
Abscission: With the onset of winter season the leaves of deciduous plants turn yellow and fall off. The most interesting aspect of leaves falling of is that most of the leaf cell materials are transferred to stems. The falling of the leaves is due to the formation of abscission layers at the base of the petioles. This layer consists of thin walled but suberized cells. The middle wall gets disintegrated and the leaves fall down. The development of this layer is stimulated by a plant hormone called ABA. But the application of auxin to such leaves prevents the formation of abscission layer and thus prevent the falling off the leaves. This has a greater application particularly in preventing the premature falling of fruits like apple, orange etc.
Parthenocarpy: Development of fruits without fertilization is referred to as parthenocarpy and such fruits are seedless. Normally plants do not produce parthenocarpic fruits.
But the application of auxin to flowers induces parthenocarpic fruit setting and prevents premature falling of fruits as well. Plants with triploid and many polyploid genomes do not set fruits. If such plants are sprayed with auxins at the time of flowering, fruit setting is enhanced. The fruits thus developed are large, seedless, and sweeter. Seedless oranges, lime, watermelon, sapota, ramphal and seethaphal, if they are produced this way, they have good market at the commercial level.

Gibberellic Acid
Gibberellic Acid is another natural Plant hormone found distributed in higher plants. This was first discovered in Japan, later its structural and functional details have been elucidated by the people in the west. So far 52 or more different kinds of Gibberellins have been identified. Among them GA2 2GA1 are the most common ones found in all plants.
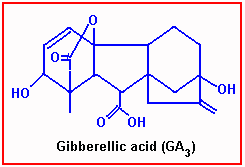
GA is a steroid like molecule in its structure. Young leaves are the sites in which this hormone is produced by phytochromes and then it is transported to the regions of growth.
The most significant effect of GA on the growth of plants is it effects internodal cell elongation profoundly than any other hormones. Even genetic dwarfs (eg. Dwarf Pisum sativum) on spraying GA on them grow as tall as the natural tall plants.
It also brings about a dramatic effect called Bolting and Flowering. Long day plants (which require 12-14 hrs of day light treatment for flowering) under short day conditions produce a cluster of leaves on highly shortened stem and never produce flowers. When such plants are sprayed with GA, the stem starts elongation with enhanced growth of internodes. Simultaneously the terminal buds start producing floral branches. This phenomenon is called bolting and flowering. However, both bolting and flowering are separated by time and space.
Besides the above said effects, GA is essential for the mobilization of food reserves for the germinating cereal grains like wheat and maize. At the time of germination, the young embryonic axis synthesizes this hormone, which is soon released into endosperm and reaches the Aleurone layer. It is in this layer; GA activates differential gene expression. As a result, specific m. RNAs are transcribed and later specific proteins like amylase are synthesized on translation. The amylase thus synthesized, acts upon the starch found in the endosperm, and the glucose thus released is drawn into the embryonic tissue which is in dire need of energy source.
Notwithstanding the above effects, GA also effects parthenocarpy, rooting, germination etc. This hormone also interacts with other natural hormones like auxin and cytokinin and brings about differential growth.
Cytokinin:
Another class of growth regulating hormones was discovered by Miller et al and they are called Cytokinins. They are the derivatives of nucleotides. 6-Furfuryl amino purines, Benzyl amino purines and such compounds act as cytokinins.
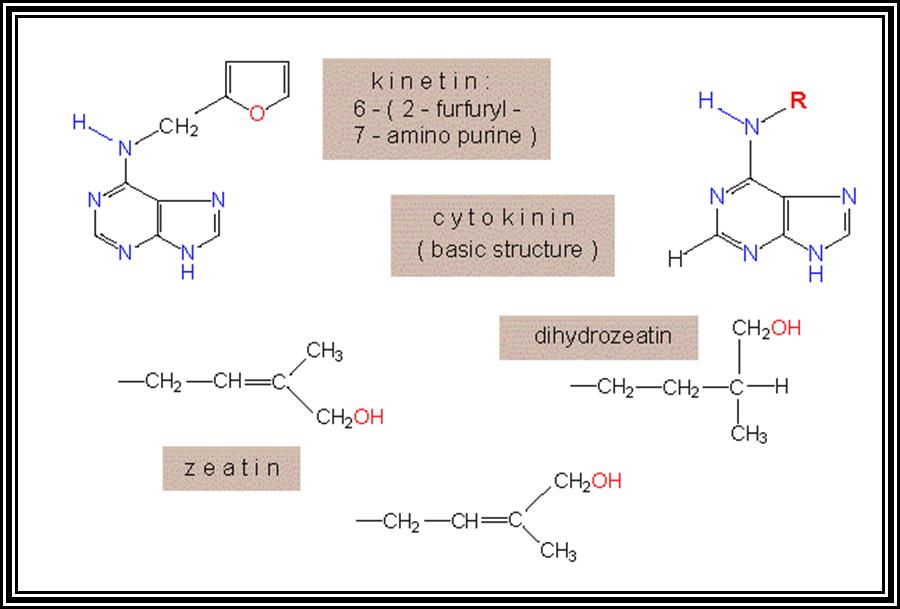
Cytokinin, are generally synthesized in the root tips, and then they are translocated to other regions. The endosperm of coconut milk, Borassus and of many other plants is a rich source of cytokinins. Cytokinin level in plants is further regulated by other hormones like Auxins and GAs. On the contrary cytokinins control the synthesis of other hormones like Abscisic acid, a growth inhibitor.
Cytokinin perse has an important role in andcytokinesis. But along with auxins, it effects differentiation of plant organs from the callus. Cytokinin by itself inhibits root formation, but in the presence of auxins, it induces organ formation like roots and shoots. If the ratio between auxin and cytokinin is high, roots are differentiated, but the lower ratio induces the formation of shoots. The intermediate ratio stimulates the development of these hormones in differentiation at molecular level and it is very interesting but unfortunately it is least understood.
Cytokinins are also found to break dormancy of winter buds as well seeds. The exact mechanism by means of which they over come the dormancy is not known, still it has been thought, that they effect through the suppression of Absciccins which is responsible for dormancy. Another interesting effect that the cytokinins produce is the prolongation of senescence (Ageing). This effect is often referred to as Richmond and Lang’s effect. This process has many implications in horticulture and agriculture, but not much attention has been given to this area of research.
ABSCISSIC ACID (ABA)
Abscisins were first isolated from dormant winter buds; they were then called as Dormins. Later the chemical studies showed that the Dormins are nothing but Abscissic acids. They are mostly found accumulated in dormant buds and seeds. They can be induced to synthesize and accumulate by providing unfavorable conditions like drought or very cold conditions.
Effects: Abscissic acid is known to be a growth inhibiting hormone. It does so by inhibiting protein synthesis and dehydration processes. Abscissic acid also induces abscission layer formation in the stalks of fruits and leaves under certain conditions. ABA's effects on turnout movement of guard cells and other turnout movements are well known. Even geotropic curvature of root is attributed to the action of ABA.
ETHYLENE
Probably Ethylene is the only known hormone that exists in gaseous state. It is naturally produced in plant parts under certain conditions. Particularly, in the presence of high concentration of auxins, ethylene production increases.

Effect: Ethylene can induce abscission layer formation similar to ABA. Some times its effects on roots are perplexing and render them insensitive to geotropic effects and they curl upwards.
Fruit ripening: The most important effect of ethylene is on fruit ripening. The effect of ethylene on ripening is very dramatic.
Ethylene, to begin with enhances the rate of respiration tremendously. This type of respiration is cyanide insensitive. At the same time, it also activates other metabolic processes leading to the degradation of organic matter and converting the same into sucrose and organic acids. It also activates certain genes required for pectinase, cellulase and other enzymes required for anthocyanin pigments.
The hydrolyzing enzymes degrade the middle wall and even some cellulose gets degraded. At the same time the green color of the fruit wall is replaced by coloring pigments like anthocyanins. All these biochemical reactions are cascade of events and ultimately all the processes reach a peak state, which is considered as Climactric state. The end result is that the hard and sour fruit turns into soft and sweet fruit
Application of Plant hormones in Agriculture and Horticulture:
Plant hormones, because of their different effects on plant growth and differentiation, have been in use in the fields of horticulture and agriculture. The following uses have been listed.
1. Auxins and Gibberellins are used in inducing parthenocarpy. Parthenocarpic fruits are seedless, at the same time they are larger and sweetish. These qualities of fruits have greater commercial value.
2. Spraying of auxins to fruit plants like orange, lemon apple etc prevents the premature falling of the fruits by formation of abscission layers, premature falling cause greater economic loss to cultivators. This can be prevented by the use of auxins.
3. Some of the synthetic auxins can be used in removing weeds growing in the farms and agricultural fields. Certain hormones specifically weed out monocots, like grasses and some destroy dicots, for ex. 2.2. Dichloro propionic acid removes grasses, while 2, 4-Dichlorophenoxy acetic acid (2, 4-D) & (2, 4, 5 Trichloro phenoxy acetic acid) 2, 4, 5-T) are employed in weeding out the dicots.
4. Auxins are effectively used in rooting of difficult to root plants. This has a wide application in vegetative propagation of plants.
5. Auxins like Naphthalene acetic acid (NAA) are used in prolonging the dormancy period, thus the storage and shelf life of food in potatoes, corms, bulbs, etc., is prolonged.
6. Synthetic as well as extracted natural hormones are extensively used in the propagation of various horticultural, silvicultural plants through tissue culture techniques.
7) Abscissic acid can be used to obtain uniform harvesting of citrus fruits and cotton balls.
8) Ethylene can be used for quick ripening of fruits for marketing.
PLANT MOVEMENTS:
In response to various environmental factors, or by themselves plants exhibit various types of movements. Many of these movements are autonomous in nature and others are in response to external stimuli like light, soil, water, touch and chemicals. Any of the above-mentioned agents which induce certain changes in the plants is referred to as stimulants. Reaction of the plant body to such stimulus is called reaction of the plant body to such stimulus is called response. Neither all parts of the plants are involved in receiving the stimulus, nor do all plants respond to the given stimulus. In many cases the region that is sensitive to stimulus is called the region of perception, which is separated from the region of response; sometimes both may occur at the same site.
For a proper response, the stimulus should be applied for a certain period of time below which the response will not be fully achieved. Such period is called presentation time. The presentation time of the stimulus is provided with optimum dosage. Nonetheless, once the stimulus is provided, the plant body after absorbing the stimulus provided, reacts to it and responds to it. The time between the application of stimulus and response is called reaction-time or latent time. If the stimulus is provided without any time for relaxation, the plants start responding weakly and finally they don’t show any response. This state is called Fatigue. The extreme fatigue leads to a condition called ‘tetanus”.
Types of Movements: Movements in plants can be broadly classified into physical movements and vital movements. In the case of physical movements, the physical forces act upon the nonliving structures of the plant parts and as a response, these structures exhibit certain movements. On the other hand, living cells of plant body are involved, in movements which are called vital movements. Again, basing on the type of movements involved, the vital movements are further classified as shown in the chart.
![]() Movements
Movements
![]()
Physical Vital
![]()
Movements of Movement of
![]() Locomotion curvature
Locomotion curvature
![]()
![]()
Paratonic or Autonomic Autonomic Paratonic
Tactic movements movements
![]()
Nastic Tropic
(Non-directional) (Directional)
Physical: Elaters of Bryophytes, dehiscence of capsules, opening and closing of peristomial teeth in Moss capsules, bursting of capsules, swelling of seeds, etc are the few examples for physical movements which respond to the changes in the moisture content. Most of the structures mentioned above are made up of lignin or such hydrophilic substrates. These substances adsorb water and swell or lose water and shrink. This expansion and contraction bring about the movements, sometimes with considerable force and violence.
Vital Movements:
I (a) Movements of locomotion: Autonomic type: These movements are basically the movements caused by protoplasmic activity. They are a kind of involuntary types that go on all the time. Nevertheless, their movement is an active process requiring the utilization of metabolic energy (ATP). They also respond to certain factors like temperature, respiratory inhibitors and growth hormones. Ciliary movement, amoeboid movement, gliding movement and protoplasmic streaming (cyclosis and rotation) movements are the examples of this kind.
I (b) Movements of Locomotion: Paratonic type;
These are induced movements, various external factors like light, chemicals, temperature act as stimuli. Accordingly, they either move towards the stimuli or move away from the stimuli. Photatactic, chemotactic and thermo tactic are the examples of this kind.
II (A) Movements of curvature: Autonomic.
Autonomic: The upward and downward movement of the basal leaflets of Indian telegraph plant (Desmodium gyrense) is a non growth movement exhibited by a rhythmic osmo-regulatory process. Nutation and Circum -nutation movements exhibited by stem tips and tendrils are the growth movements. Because of the alternate or unequal growth of the plant cell structures, they show such bending and rotatory movements.
II (B) Movements of Curvature: Paratonic type.
Paratonic movements are the induced movements, which may be directional or non directional. They may either growth movements or non growth movements.
1. Tropic Movements
The growth movement in response to external stimuli, like light, gravitation, water and touch are called phototropism, geotropism, hydrotropism and thigmotropism respectively.
Phototropism: The bending movement of stem tips towards the light source is called phototropism. Here the light provides the stimulus. The actinic part of the white light that causes the positive curvature movement, it is the blue part of it. This indicates that the plant cells which respond to this light must possess a pigment system which is capable of absorbing the blue light. This pigment has been identified as riboflavin and it is called as “cryptochrome”. How this pigment brings about the differential growth, which is responsible for the growth curvature, is not very clear. Added to this, the involvement of auxin in the growth/ elongation of the cells is of prime importance. When the stem tips are exposed to unilateral light, it has been found that the Auxin gets distributed unequally between the cells exposed to light and the cells in the unexposed area. This unequal distribution (i.e., more in the unexposed area and less in the exposed cells), brings about more growth in the cells containing more auxin and relatively less growth in the cells with less auxin. As a result of this the stem bends towards light. Whether the unequal distribution of auxin in the cells caused by sunlight is due to the lateral transportation from the illuminated area to darker area or due to inactivation of auxin found in the cells of illuminated area is a muted question. Recent experiments indicate that the active form of auxins found in the cells of the illuminated area become inactive form rather than the complete destruction. This change in active form is very important. More over, the exposure of cells to unilateral light may also cause the synthesis of ABA locally and it may be responsible for the inhibition of growth of the cells. This may be achieved by extrusion of K ions from the cells of illuminated area to other side and make the cells flaccid or it may bring about a change in active form of auxin to inactive form.
A new dimension has been added to this phenomenon with the observation that the treatment of stem tips with 0.5% colchicines, makes the stem tip insensitive to either light mediated or auxin mediated growth curvature. This finding again supports the idea of auxin bringing about the growth effect by activating the tubulin polymerizing system. However, the exact mechanism is still not very clear though we have some facts and figures of observed in growth movements.
Geotropism (Barytropism or Gravitropism): When a seedling is kept horizontally on the soil, the radicle always grows towards soil, and the plumule grows away from the soil. This type of growth movement of radicle and plumule is called positively geotropic and negatively geotropic respectively. But some runners and rhizomes grow parallel to the surface of the soil and this is referred to as Diagiotropic. When the lateral roots grow obliquely into the soil, it is referred to as plagiogeotropic phenomenon.
Sites of Geotropic Perceptions: In stem, the maximum perception is received by stem tip, but in root, it is the root cap that acts as the site of perception.
Mechanism: The analysis of auxin distribution in the plant as shown in the figure indicates that stem tip contains highest concentration and the root tip has next highest. This suggests that the optimal growth of stem tip requires higher concentration and root tip requires lower concentration for the maximal growth. On the other hand, it can be stated that the concentration required for the maximum growth in stem tip is inhibitory to the growth of root tip, but the concentration that is optimal for root tip is inadequate for the maximal growth of the stem tip.
When a seedling is placed horizontally on the surface of the soil the auxin moves down by gravitational force and accumulates in cells that are found facing the soil. The stem apex and root apex respond differently to different concentration of auxins. Higher concentration in the stem tip enhances the growth of the cells which have received more auxins. So, the upward growth curvature occurs. On the contrary the growth in the root apex is inhibited because of higher concentration, but lower concentration in the upper region promotes the cell growth, hence the roots exhibit downward growth curvature.
This differential growth behavior of stem apex and root apex to different concentration can be further sustained by planting the seedling in a rotating pot of the Clinostat, so as to make the auxin concentration uniformly distributed. Because of this, root and stem grow straight with out any curvature.
The above observations were found to be inadequate for explaining geotropic movement. Nevertheless, Berthelot and Haberlandt suggested that starch containing amyloplasts act as statoliths. When root is placed horizontally, the statoliths because of their weight are displaced from the original position and move downwards to settle on the membranes below. This contact of statoliths with cell membrane of that side causes irritation and inhibits cell growth on that side, but other side of the cell grows normally. Unfortunately, this theory failed to take into consideration of those plants which are devoid of statoliths but still showing geotropic response (Eg. Fungi etc).
Recently it has been demonstrated the root cap has a profound effect on the geotropic curvature, because the root caps synthesize IBA and release it to the lower cells. This transport of IBA causes the inhibition of cell growth in that region and other cells which are free from IBA grow normally and exhibit growth curvature. Still, it is difficult to visualize how IBA brings about this effect. Nonetheless, one thing is clear that the cells in stem tip and root tips though totipotent, their potentiality to receive stimulus is different and the mechanisms they operate are also different. To achieve the growth curvature, they use their own phytohormones.
Hydrotropism: Whatever may be the position or direction in which the seedling is placed in an inert vermiculite or saw dust after providing water from the base, the radicle always grows / towards water. This type of growth movement is called hydrotropism. The exact mechanism by which the root tip seeks water, as it grows towards soil, is not known except for the fact that roots have in-built potentially for absorption of water and mineral salts. It is the innate desire that drives roots to grow towards water. Physiological mechanisms that generate these movements are not known.
Thigmotropism: Certain parts of the plant body like tendrils etc., are highly sensitive to touch. These organs are found to have tactile pits for the perception of the stimulus. When cells come to contact with the surfaces like wood, stems, etc., get irritated and synthesize and liberate IBA. This causes the inhibition of growth of these cells at that surface while other cells which are away from the contact region continue to grow normally. Thus, they bring about the coiling of the tendrils around the supporting structure.
2. Paratonic Nastic Movements: The movements are a kind of induced movements, but these may be also due to growth or osmoregulation.
Photonasty: This type of movement is induced by light and darkness. Photonastic movements otherwise called Nyctinastic or sleeping movements are exhibited by the leaves of many leguminous plants where basal petioles of the leaflets have a bulbous structure called pulvinus. Here the leaflets either fold upwards or downwards. The mechanism responsible for this type of movement is believed to be due to turgour changes in response to blue part of the white light. The exact mechanism of differential turgour changes that occur in upper and lower cells of the pulvinus is not known. However, it is suspected that K ion influx and efflux is responsible for such turgour movements.
Chemonasty: Insectivorous plants like Drosera, Nepenthes, Dionea, exhibit this type of chemonastic movements. These plants grow in N2 difficient areas and require nitrogenous products for their sustenance. Hence these plants have devised various insect trapping mechanisms in which the specialized structures are well developed. When an insect, mistaking for glistening honey or attractive food comes in contact with the perception sensitive sites, the hairs which are sensitive pass on the stimulus to hinge part of the leaf, which immediately responds in closing the leaves over the prey. This excitation, conduction of excitation and folding movement is due to ions influx and efflux, which actually cause turgour movements.
Seismonasty: This is brought about by mechanical or seismonastic irritability in the force of touch. Mimosa pudica, Biophytum sensitivum and others exhibit this type of movement. The leaf pulvinae of these plants are made up a central vascular strand surrounded by a large area of cortical cells, with large intercellular spaces. The cortical cells found in the lower part of the basal pulvinus of the leaves have thinner cell walls than the upper cells. These thin-walled cells are highly sensitive and collapsible.
When the terminal pinnae of the bipinnate leaves of Mimosa pudica are stimulated by touch (mechanical irritability), the stimulus is transmitted from the tip of the base at the speed of 10-20 cm/sec. This is virtually similar to that of the conduction of nerve impulse. Once the stimulus is conducted to the pulvinus, the upper thin-walled cortical cells collapse by losing cell water into intercellular spaces. This results in the upward bending movement and pinnae close. When the stimulus reaches base of the leaf the cortical cells found in the lower part collapse by the same process and the entire that are released in the perception of stimulus and the chemicals that are transmitted all along the length of the xylem elements are not clearly known. However, it has been suspected that the mechanical shock or stress releases the IBA, which in turn sets on the efflux of K ions in the cortical cells, which is actually responsible for the collapsing of the cells and the movement. The leaves take 30 minutes to one hour to recover from this shock.