PLANT AND WATER RELATIONSHIP
Water being considered as an universal solvent, occupies 75% of our planet in the form of oceans. Added to this water is also found in the atmosphere in the form of Hydrospheric mantle. The evaporation of water from the surface of ocean, formation clouds and raining, is a natural cycle evolved during course of Evolution of this planet. Nearly 3.8 billion years ago, life took its origin as a speck of protoplasm in the churning oceanic water which was not salty as it is today. In the course of Chemical Evolution, the birth of life has taken place in H2O, it is the medium of biochemical activities. Thus, water has become mother of life or “Solvent of Life”.
Cells of all organisms are made up 90% or more of water. And all other components are either dissolved or suspended in water to form protoplasm, which is often referred to as physical basis of life.
Importance of water: Water is the major component of living cells and constitutes more than 90% of protoplasm by volume and weight.
It acts as medium for all biochemical reaction that takes place in the cell and also acts as the medium of transportation from one region to another region. Water is a remarkable compound, made up of Hydrogen and Oxygen (2:1) and it has high specific heat, high heat of vaporization, high heat of fusion and expansion (colligative properties). Water because of its bipolar nature acts as universal solvent for it dissolves more substances than any other solvent. Electrolytes and non-electrolytes like sugars, and proteins dissolve very well. Even some hydrophobic lipid molecules show some solubility in water.
Water acts as a good buffer against changes in the Hydrogen ion concentration (pH). This is because of its ionization property. Certain xerophytes use water as buffer system against high temperature.
Water also exhibits viscosity and adhesive properties. Because of hydrogen bonds, water molecules are attracted towards each other, they are held to each other with considerable force. This force of attraction is called cohesive force. Thus, water possesses a high tensile strength. If this water is confined in very narrow columns of dimensions such as xylem vessels, its tensile and cohesive forces reach very high values (1000-1200 Gms). And this force is very helpful in ascent of sap. Water is of great importance in osmoregulation, particularly in the maintenance of turgidity of cells, opening and closing of stomata and growth of the plant body.
Water is an important substrate in photosynthesis, for it provides reducing power for CO2 fixation; water is also used in breaking or making chemical bonds of polypeptides, poly-nucleotides, carbohydrates etc.
All the above features clearly indicate that water plays an most important role in the regulation of life processes.
Diffusion
If the scent is sprayed in one corner of the room, the smell spreads to all part of the house in no time. If a fire wood is burnt, the black soot goes up and spreads. If a pinch of solid potassium permanganate if dropped into water contained in a beaker, pink color slowly diffuses and spreads throughout. The above said spreading phenomenon is due to movement of molecules. Having their own kinetic energy, water molecules will be in constant motion randomly.
Diffusion is governed Ficks First Law. It depends upon the rate of transport or Flux density-ds, s-is the substance crossing over a unit area per unit time. Diffusion coefficient (Ds), it is proportionality constant Ds that measures how easily a substance moves through a particular medium. The concentration gradient is defined as δcs/δx.
Molecules of a particular species always tend to move or now call it diffuse from higher concentration to lower concentration irrespective of the other types of solutes present in the system; i.e., from higher kinetic energy to lower kinetic energy. However, the rate of diffusion is governed by other factors like; (1) concentration gradient (2) temperature (3) density of molecules (4) pressure and (5) medium through which it diffuses.
Water Potential (φW):
In this context, it is important to be familiar with the term called water potential (φW) which refers to the chemical free energy of water. The chemical free energy of pure water or solutes is always expressed in terms pressure units such as bars. 1 atmosphere (Atm) = 14.7 pounds per square inch, that is, = 760mm Hg at sea level, =1.013 bar, = 0.1013 Mpa, = 1.013 x 10^5 Pa (1 bar = 0.987 atmospheric units, 10 bars = 1 Mega Pascal (Mpa). 1 mpa = 106 dyne / cm2, under standard conditions). To give a common-values; for the Car-tire typically pressured at about 0.2Mpa and the water pressure less than 15 feet (5m) below; water is about 0.05Mpa.
The chemical free energy of water in its purest form is also called water potential (φW). Purest form means there are no other molecules in it. The chemical energy of it is maximum and its value is given as 0 bars. Addition of solutes to pure solvent decreases the chemical free energy of pure water, because certain amount of energy of a number of water molecules is used for binding to the surface of solutes. So, the total value of water potential in a solution is less than zero; it is always expressed in negative pressure values. Here it is equal to DPD; if the water potential of pure water is zero and DPD is also zero. But the water potential of solution is less than zero expressed in negative value, but DPD of the solution is expressed in positive value.
These energy relations are governed by the said equations (below), understanding of it is very important.
φW = φs + φp = φg
φw = water
φs = solutes-solutes potential or osmotic potential.
φp = pressure-hydrostatic pressure of the solution, it is often called turgor pressure, which can be negative or positive.
φg = gravity- will not be considered for normal calculations.
Pure water:
φp = 0 Mpa
φs = 0 Mpa
φw = φp+φs = 0 Mpa
DPD of pure water = 0 bars; DPD of a solution = (+) bars
Ψ w of pure water = 0 bars; ψ w of a solution = (-) bars

Osmosis
If two solutions of different concentrations are separated by a plasma membrane, which is semi permeable as well as selectively permeable, the solvent (in this case it is H2O) moves through the membrane from higher concentration towards lower concentration. Here the plasma membrane has a differential permeability, where it allows the diffusion of water molecules to move from higher concentration to lower concentration but it prevents the movement of solute molecules from higher concentration to lower concentration. Such differential diffusion through a semi permeable membrane is called permeability.

Osmoisis:
Osmotic potential: Osmosis is always referred to living cells. The movement of water into the cell (endosmosis) or moving out as plasmolysis, mostly depends upon the concentration of solutes which are of varied types like minerals, salts, proteins, carbohydrates, fatty acids etc., and these contribute to the osmotic potential or osmotic concentration, which contributes to the osmotic pressure. Higher the concentration of solutes, higher is the osmotic pressure and vice-versa.

A solution is made up of a solvent within which solutes are dissolved or present in soluble form. Basing on the concentration of solute in a given volume of solution, it is referred to as dilute solution or concentrated solution. The concentration of solutes in an enclosed system having a constant volume exerts a pressure because of the kinetic movement and collision of the solute molecules. The pressure that is exerted by the solute in a system, either separated or enclosed in a semi permeable membrane is called osmotic pressure. Hence a dilute solution exhibits lower osmotic pressure and
concentrated solution shows higher osmotic pressure which is also called osmotic potential.

Besides the concentration of solutes, Osmotic potential is governed by other factors of life, ionizing potential of the solutes, temperature etc.

Those solutes which undergo greater ionization (electrolytes) exert greater osmotic pressure than non-ionizing substances (non-electrolytes). Similarly, temperature has a contributing factor for osmotic pressure, because with the increase in temperature, molecular movement increases and so also the collision, hence OP (Osmotic Pressure).
Chemical free Energy of Water: Water, for that matter, any solvent in its pure state has its own chemical potential by virtue of which it exhibits random movement. This is referred to as chemical free energy or water potential. If such a solvent is separated from a solution (solvent + solute) by a semi permeable membrane, water molecules move from higher chemical or water potential to the lower water potential. In this case pure water has higher chemical energy than the solution, for the solute present in water lowers the free chemical energy of pure solvent of the solution.
Diffusion Pressure Deficit (DPD)
In a pure solvent, all molecules will be moving freely by virtue of their chemical potential. This random movement is called diffusion. It further depends upon the concentration of diffusing molecules, which in turn exert a pressure termed diffusion pressure. The direction and rate of diffusion in a pure solvent is random but equal and opposite. Hence the diffusion pressure exerted in such a system can be taken as zero. To such a system, if solute is added, it undergoes solubility, where some freely moving solvent molecules get bound to solute molecules and in fact in some cases, they form a shell around such salts. This results in the loss of considerable number of solvent molecules for free diffusion. This loss is called diffusion pressure deficit. In this process there is loss of chemical free energy of water because of the binding of solvent to solutes. Thus, the DPD is governed by the relative concentration of solute in a given volume of a solution.
Increase in the concentration of solute in a known volume of solution increases the DPD of the system. Furthermore, increase in solute’s concentration also increases OP; hence DPD and OP are related to each other. The water or solvent always moves from lower DPD to higher DPD.
All the above-mentioned phenomenon like Osmosis, OP, DP, DPD are governed by simple physical forces. Nevertheless, they play a very important role in living systems. Cell can be considered as an osmotic bag for it is bounded by cell membrane, enclosing a semi liquid cytoplasm which is made up of more than 90% of water and the rest are inorganic salts and organic molecules and cell organelles. While the cell membrane acts as osmotic membrane (semi permeable), the other organic and inorganic of the cell. Creation of differential osmotic potentials between the cells or between the environment and the cell, acts as the motive force in performing various functions like absorption of water, ascent of sap, root pressure, turgour pressure, plasmolysis, absorption of water translocation, transpiration etc.
Plasmolysis, Endosmosis and Turgidity
Exosmosis, Endosmosis and Turgidity:
When a normal cell is put in a hypertonic solution (solution with high concentration of solutes, than the solute concentration of the cells), a water potential or DPD gradient is created between the cell and the external solution. Hence the water diffuses out of the cell; the process is called Exosmosis or Plasmolysis. As a consequence, the cell collapses and the plasma membrane withdraw from the cell wall and the whole cytoplasm gets concentrated in a corner of the cell. Such a cell is called flaccid cell. In this state, turgour pressure (TP) is zero, and OP is very high, but = w.
If such a cell is transferred to hypotonic solution i.e. (the solute concentration is less than that of a cell) the cell enlarges for the water enters into the cell. If the solute concentration of the solution is equal to the cell concentration, then it is called Isotonic. If the water from external solution enters into the cell, this process is called Endosmosis or Deplasmolysis. As a result, the concentration of water or water potential within the cell increases. Increase in the water concentration into the cell creates its own molecular pressure within the cell and it is called turgour pressure. With the increase in turgour pressure, the cytoplasm swells and gradually plasma membrane is pushed towards the cell walls. As more and more water enters, more and more of turgour pressure builds up and the cell goes on increasing in the size. The water potential of the cell increases towards zero value.
As turgor pressure exerts its impact outwardly i.e., on to the cell wall, the cell wall being plastic, exerts counter pressure; this is called wall pressure. When the TP (p) becomes equal to wall pressure, the water potential within the cell and outside the cell reaches an equilibrium state. Such a cell is called turgid cell.
The relation can be expressed in the following formulae:
If DPD = OP - TP If φW = ψS+ ψp
By determining OP or P one can determine the DPD or w of any given cell. This can be done by initial plasmolytic method.
Where M = molarity at which incipient plasmolysis occurs, t = Room temperature, 273 = Absolute temperature.
= (-) mart
Where, OP = osmotic potential, M = molarity at which incipient plasmolysis takes place, I = Ionization constant i.e., one for glucose and sucrose, but for NaCl, it is two, R = gas constant, i.e., 0.083 bars, T = 273 + room temperature say 250C.
If a fresh grape of the plant is dropped into a highly concentrated sugar solution, it shrinks. For that matter, any living cell exhibits the same property. This is because the solute concentration in the external solution is greater than the solute concentration in the cells of the grapes. So, a DPD gradient is created between the cells of the grape and the external solution. As the water content in the grape cells is more than the water content in the external solution, in other words, water potential in the grape cells is higher than the water potential of the external solution, the solvent water diffuses through the membranes into the external solution. Hence, the plasma membrane withdrawn from the cell wall and the cell shrinks. This status of the cell is called flaccid and the phenomenon is called ‘Plasmolysis’.
The reverse of this phenomenon is called ‘Endosmosis’. If flaccid cells are put into a pure solvent like water, because of the DPD gradient, water moves into the cell. As water molecules diffuse into the cells, the concentration of it increases, so also the cell volume. This increase in the concentration of water creates a pressure called Turgor pressure, builds up within the cell; cell volume increases continuously till the time when the elastic wall is stretched to the maximum. At certain point the wall because of its elastic property, starts exerting counter pressure called Wall pressure. When wall pressure is equal to Turgour pressure the movement of water in and out of the cell becomes equal and this state of the cell is referred to as Turgid and the phenomenon is called Turgidity. Development of turgour pressure gives mechanical strength for the plant to stand errect. It also acts as the motive force for many functions like movement of stomata, nictinastic movement of leaves of Mimosa pudica, movement of bulliform cells of grass leaves etc. Cellular metabolism is so regulated, in response to certain stimuli (whether it is external or from within), cells develop DPD gradient, as a result, water moves into such cells and brings about turgidity which results in the movement.
Hydrophilic substances like polysaccharides, proteins etc. of cell walls and storage tissues attract dipolar water to them. Water molecules in turn bind to the charged surfaces. As a consequence, the imbibing cells, swells in volume; such a phenomenon is called imbibition and the pressure generated due to imbibition i.e., in the form of swelling force is called ‘Imbibition pressure. During this process some amount of energy is lost and it is called imbibitional energy.
In many cases the imbibition force developed due to the imbibition of water is very high (ranges from 1000 to 10000 bars). The same can be used for breaking big boulders in queries. Even today this method is in practice.
ABSORPTION OF WATER:
Water in the soil is mostly and abundantly, under normal conditions, is available in the form of Capillary water. In the soil the space in between soil particle forms a network of spaces, which normally is filled with water. The water that is present in such spaces is called capillary water.
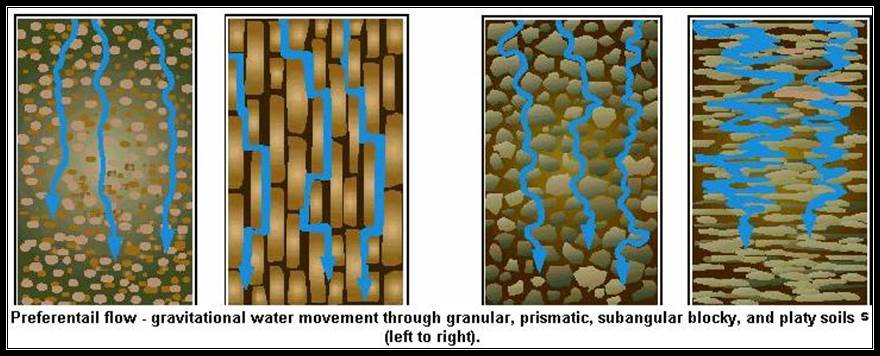
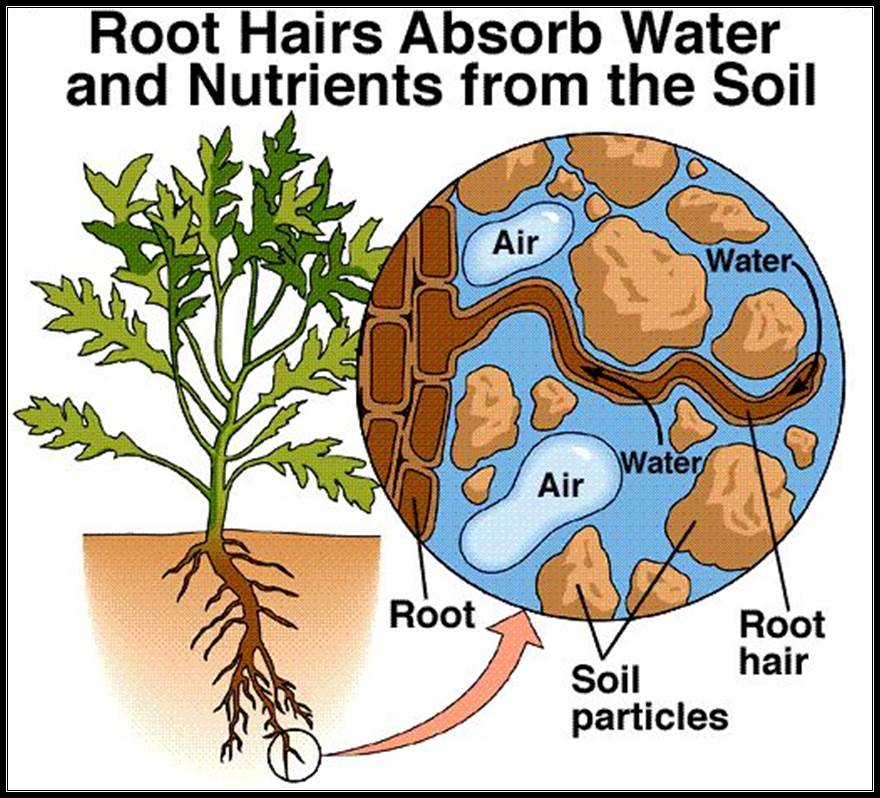
Structures involved in Absorption:
The root terminal region is made up various structures such as; from the tip towards base, apical meristem, zone of elongation, root hair zone and zone of maturation. The root hair zone is studded with root hairs; they are the extensions of epidermal cells in the form of tubular structures.

Most of the water is absorbed by the plants is through root hair zone. The figure shows the pathway of soil water into root system.

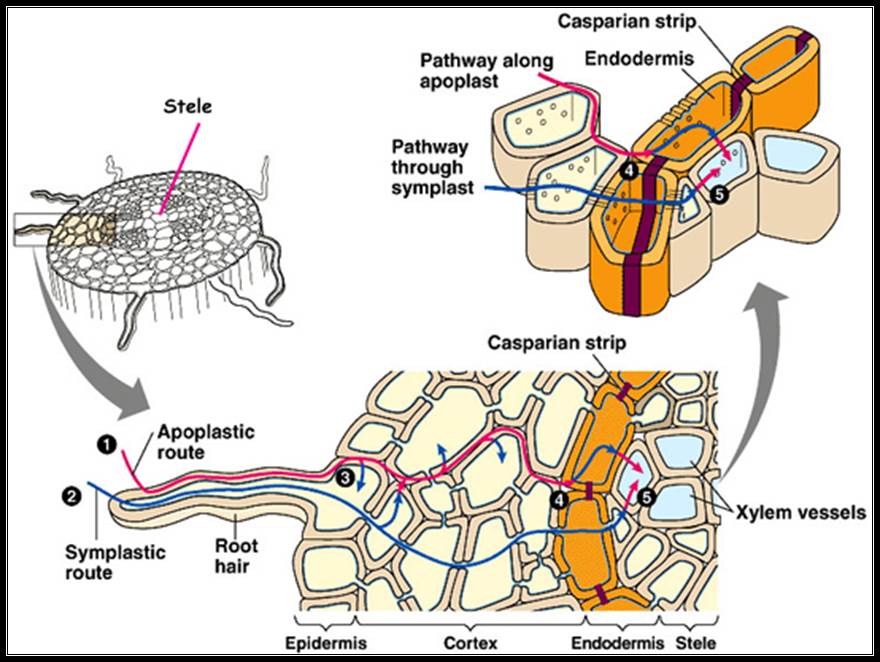

Apo plastic movement of water; that is the water moves through the space found in the cell wall.

TRANSLOCATION OF WATER OR ASCENT OF SAP
Water that is absorbed by roots has to be transported to the terminal regions of plants. This movement of water is called Ascent of Sap or Translocation of Water.
Structures involved in Ascent of Sap: Various experiments like girdling, staining and plugging, indicate that the xylem tissue is mainly responsible for the movement of water. As xylem consists of tracheae otherwise called vessels, they form a system of fine channels running from roots to all other regions of the plant body and form a beautifully branched supply system, which is almost similar to that of arteries in animals.




Rate of direction of transportation: The rate with which the water is transported along the length of the stem varies from plant of plant. External conditions also play a significant role in controlling the rate of ascent of sap. But, under normal conditions the rate is 75-100 cm/hr. This is quite a rapid process. Generally, most of the water is translocated upwards i.e., in longitudinal direction, but water is also translocated horizontally to reach the peripheral tissues.
Vital Theories: These theories are mostly based on the assumption that living cells play a vital role in pushing or pumping the water upwards. Westermaier (1883-1884), Godlewski (1884), Janse (1887) and others thought that the xylem vessels through which water is transported would just act as vessels through which water is transported would just act as reservoir. In this method both living and nonliving xylem forms an integrated system and act co-ordinatingly in the transport of water upwards. Unfortunately, these theories had little experimental evidence to substantiate their claims.
Sir J.C. Bose (1928-29) the elder brother of Subash Chandra Bose (S.C.Bose), proposed an interesting theory called ‘Pulsation theory’. His theory was based on the assumption that living cells all-round the xylem tissues are in a kind of rhythmic contraction and expansion, similar to that of heart in animals. This rhythmic pulsation is responsible for the movement of an instrument ‘Cresograph’ where an electrode is inserted deep into the plant stem and the pulsations were graphically recorded. The oscillations found on the graph indicated the pulsation activity of the cells. A little later Molisch, (1928-29), provided example, he injected some heart stimulants into stems and demonstrated an increased pulsation activity. On the contrary anesthetics brought down the pulsation rhythm.
Recent investigations however, using respiratory inhibitors like DNP, KCN, showed that the synthesis of ATP is essential for the rapid other islets of living cells intermixed with the longitudinal array of trachieds and tracheae. These strongly support the view that vital activity, in the sense, the energy is required for the ascent of sap.
Root pressure theory: Absorption of water by roots has been mainly a passive process. However, the involvement of an active process is not ruled out totally. On a rainy day, when the atmospheric humidity is at its maximum, and transpiration is at its minimum, root system absorbs excess of water than it can normally absorb. As a result of it, hydrostatic pressure builds up within the roots, and this is called root pressure. This is believed to act as the motive force to force the water into the xylem columns upwards. Under the above said environmental conditions, water is forced out of the water-stomata as guttated water. Hence root pressure has been considered as an important phenomenon in ascent of sap. However, it has been noted that, some of the tallest trees found on this planet do not show any root pressure. Thus, this theory fails to explain the transportation of water especially in tall trees.
Passive or Physical Force Theories: Physical forces like capillary force, collision force, atmospheric pressure, imbibitions, diffusion pressure, are found to operate in plants in one way or the other. Along with the development of science of plant physiology, people from time to time have come out with various theories involving one or to time have come out with various theories involving one or the other physical force as an explanation for ascent of sap.
Atmospheric pressure theory: the protagonists of this theory have assumed that plants are closed systems. When water escapes by transpiration from the surface of the leaves, it is believed that vacuum will be created within the plant body. As the root system is submerged in soil water, with the atmospheric action on the soil water, in order to fill up the vacuum created in the xylem vessels, water just enters passively; thus, the water is translocated upwards. Unfortunately, plants are not closed systems but they exhibit openness, for, the gases can diffuse into and out of the plant system with ease and facility. Added to this, atmospheric pressure can support and facilitate this process. Added to this, atmospheric pressure can support the water to be lifted only to a height of 34 feet; but there are plants which are taller than this and still there is transport of water. Hence it can be concluded that atmospheric pressure could not be the force for ascent of sap, but transpiration of water facilitates this process.
Capillary Force Theory: When one end of the blotting paper or a chalk piece is dipped into ink, the ink slowly moves up. This movement through the paper is called capillary movement. Blotting paper is made up of innumerable cellulose fibres interwoven into a close network. Between such fibres, extremely narrow spaces are found, which are connected with each other and form a fine net work of capillary canals. If water is provided to such capillary system at one end, water is sucked in and it moves along the channels of capillary network by a force called capillary force. According to capillary network by a force theory, such capillary system exists within the plant body. Tracheids and tracheae which are found longitudinally oriented in the vasculature have lumen as empty space, roots to terminal regions of the stem as continuous capillary system. When water is absorbed by the root system, the capillary system of xylem elements takes up the water by capillary force and the water is supported to move upwards slowly but steadily.

Though the network of xylem elements can be compared to capillary system, the rate of movement of water in a capillary system is extremely slow in comparison to the actual rate of ascent of sap observed. Furthermore, the lumen of larger number of tracheids and tracheae has diameters greater than the large capillary spaces. Though capillary system may be contributing some force for the movement of water upwards, it cannot be considered as the sole force for the movement of water.
Imbibition theory: Dry wood when soaked in water swells; wooden shutters in rainy season are difficult to close; seeds soaked in water overnight get swollen. In all the above said instances the volume of the wood or seeds increases. This is due to the intake of water into the dry plant material. Wood is made up of cellulose, hemicelluloses and lignin. All these substances are hydrophilic or water loving in nature. When such materials come in contact with water, the hydrophilic substances imbibe water, which gets absorbed onto them. Because of the adsorption of water, the volume of the wood increases, simultaneously lot of energy is liberated. This phenomenon is called imbibition. If such materials are kept in a closed container and water is added, wood imbibes water and swells. This swelling creates enormous pressure ranging from 1000 to 10,000 amphospheres. People (stone breakers) using this natural phenomenon break open big stone boulders by inserting dry wooden pegs into holes, then adding water in the holes. As wooden pegs imbibe water, enormous amount of imbibition pressure develops, which is really responsible for breaking the rocks.
Plants, being made up of cellulose cell walls, do exhibit imbibition. This is particularly conspicuous with xylem elements because their thick walls are made up of hydrophilic substances. When roots absorb water, xylem cells do imbibe water and there is no doubt about it; but the movement of water along the cell wall of these dead xylem water along the cell wall of these dead xylem elements seems to be incredible, but slow comparatively. Instead of water moving along the cell wall it has been found that water moves through the lumen of the xylem elements and this is a fact. The contribution of imbibition force in the movement of water upwards is very little and negligible and it cannot be considered as the mechanism of ascent of sap.
Cohesion / Transpiration pull theory: Molecules of similar kind are attracted towards each other and they are held together by a force of attraction called cohesion force. Water, oil, alcohol etc., depending upon the kind of the solvent molecules the force of attraction varies. In the case of water, hydrogen and ionic bonds are the forces of attraction, while in oils the hydrophobic bonds are the forces of attraction. When such solvents are put into very fine tube similar to the size of tracheae, the column of the solvent, here it is water, does not break, because of two forces acting up on such column of water, one is the cohesive force and the other is surface tension.
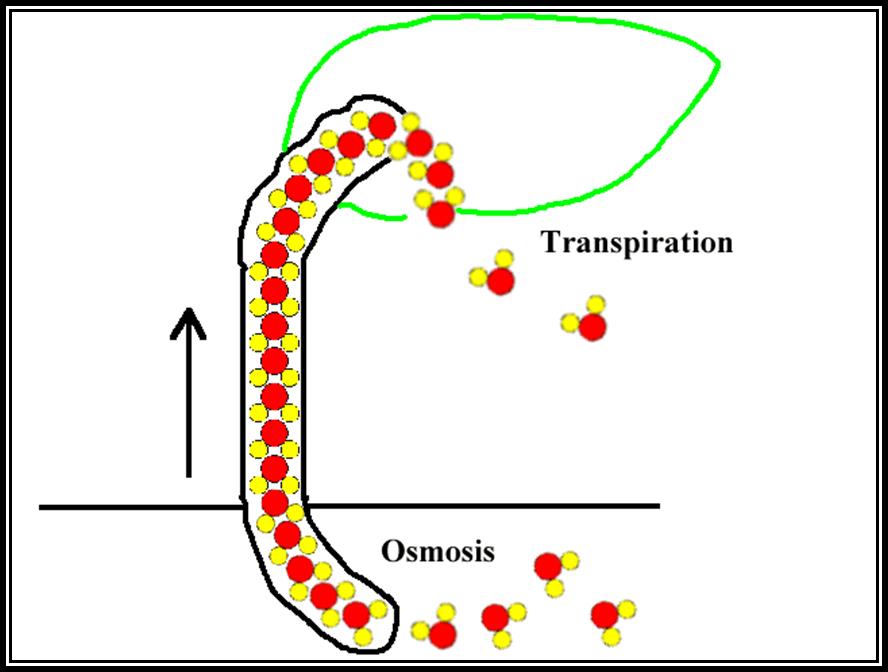
The cohesive force and the other is surface tension. The cohesive force that develops in such a column is enormous ranging from 100-1000 ATM.

Plants contain a series of such xylem vessels connected to one another forming a continuous column of cells, form basal part of the roots to the terminal leaves at the extreme apex on the stem. The water found in such long columns is tenaciously held to the walls of the vessels, at the same time the column withstands the opposite pulls by its cohesive forces. When water is transpired from the surface cells of the leaf, water is drawn from the neighboring cells due to the development of DPD gradient between the transpiring surface cells and the inner cells. This sets up a chain reaction, and xylem elements filled with water. This leads to the diffusion of water from xylem elements found in ‘lumen’ into mesophyll cells towards the anterior surface. When millions of such cells are engaged in losing water by transpiration, enormous amount of DPD gradient develops which may amount for 100-200 atmospheres.


The totality of the force is so great on the water column found in xylem elements; the water is physically pulled upwards to meet the demand of the transpiring cells. This pull or force that drains the water is called transpiration pull or suction pressure. This kind of transpiration pull on the column upwards creates a kind of tension on the water column for the water column is also pulled downwards by gravitational force. Under these conditions, forces which have greater strength prevail and the water column is pulled towards that end. In this case, the transpiration pull is much more than the gravitation pull, hence the water is pulled upwards, provided that water column has to have strength or force of attraction to withstand tension. Fortunately, the cohesive forces between the water molecules are so great, they can withstand this tension. Thus water moves upwards in column as if there is a pump sucking the water upwards. Because of such tension the cells shrink, rather reduce their lumen, when the entire stem is taken into consideration. The stem exhibits a slight narrowing during maximum transpiration. These daily rhythmic movements of expansion (night) or contraction (day) has been demonstrated by Mc Dougal. One atmospheric pressure of transpiration pull is enough to pull the water unto the height of 20 feet or so; but normally, the transpiration pull that develops runs to about 20-100 atm, which is enough to pull the water to the height of 400 to 1000 ft and the tallest plant known to mankind is just about 400 ft. Certain objections, like air bubble in water column, are taken care of by this ‘Cohesion’ theory by Dixon and Jolly (1894).

Thus it can be concluded that of all the physical phenomenon involved in ascent of sap, the cohesive-transpiration pull is the main mechanism responsible for the movement of water upwards. That does not preclude the participation of other forces like imbibition pressure, capillary force, root pressure, but their contribution is very negligible. Still one should not forget that plant is living system. It uses the non-living structures like xylem elements for the transportation of water. Thus living cells around the dead cells are absolutely essential to keep this phenomenon going; without this no plant can either survive or performs the process. Hence, the vital forces also have a say in this process.
Absorption of Mineral Nutrients:
Soil is made up of various kinds of particles all made up of inorganic minerals or mixed with organic matter. Soil contains, in general 45% of minerals, 25% or more of water, 25% of air and the rest is all organic matter. The abundance mineral matter is shown in the following table under.
Among many structures found at the root tip, the most important structure involved in absorption of minerals in the meristematic zone. Though one cannot rule out the movement of minerals in the other regions passively into Apoplastic space, but to enter into protoplasm they have to cross the barrier that is plasma membrane. Plasma membrane is studded with aquaporins for the movement of water and it also contains a variety of transporters, some exhibit what is called passive, but facilitated and others show active transport, which requires the expenditure of ATP.




TRANSLOCATION OF MINERALS
Transpiration pull is so great in its magnitude; the water is virtually sucked from the soil solution through the roots passively. As root tips are also involved in absorption of mineral nutrients, they are actively transported and emptied into xylem elements. Along with water, mineral salts arc also translocated enmass all along the transpiration stream. The same are distributed to the aerial regions with ease and facility. Thus, translocation of water and minerals takes place simultaneously in these structures and by the same forces.
Translocation of inorganic solutes
Absorbed mineral salts are drawn into vascular bundles. Particularly in xylem elements the inorganic nutrients move upwards along with water column by cohesion-transpiration pull mechanism. However, the lateral transport of food materials is an active process.
3. Plant water relationship
Water absorbed by the root system is transported upwards and the same is always lost from the aerial surfaces of the plant body. In fact, loss of water facilitates the absorption and translocation of water and minerals in the plant body.
If water is lost in the form of liquid, it is called Guttation; on the contrary if water is lost in the form of water vapors, it is considered as Transpiration.
GUTTATION
The loss of liquid water through special structures called Hydathodes or water stomata is called Guttation.
Hydathodes are normally found at the margins, the serrated margins or the acute tips of leaves. Guttation is observed in some plants ex: Colocasia, Tomato, Potato and some grasses etc., numbering about 200 - 250 species.
Contents of guttated water: The guttated water is not pure but contains organic acids including amino acids, proteins, mineral salts and even some enzymes. If such liquid drops at the tips dry out, the tips show burnt appearance, called guttation burn. The guttated water drops are different from dew drops. The dew drops are condensed pure water.
Hydathodes: Hydathodes are simple openings found in the epidermal layers. Just behind the pore an air cavity is present, which is surrounded by parenchymatous tissues called Epithem. The cells of it are thin walled, spherical and loosely arranged. The xylem elements of veins terminate in Epithem. Some of the parenchymatous cells surrounding the xylem elements are specialized and show characteristic features of transfer cells.
MECHANISM OF GUTTATION:
Guttation takes place only on a heavy rainy day with very high relative humidity in the atmosphere. Under such conditions the roots absorb more water and develop root pressure. It is the root pressure that is responsible for pushing the water up and out of Hydathodes. Thus process is considered as an active process because the development of root pressure is an active process (ATP dependent).

Guttation is found only in those plants which also develop root pressure under favorable conditions mentioned above. However, the amount of water lost by guttation is not that significant.


TRANSPIRATION
Magnitude of Transpiration: Most of the terrestrial plants absorb water from the soil and transport it to aerial regions of the plant body. Though water is essential and an important component of living cells, the plant body loses considerable amount of water from its surfaces in the form of water vapors. The magnitude of water lost in the form of transpiration varies from plant to plant. It has been estimated that a single corn plant may transpire about 54 x 4.5 liters of water in a growing season. On the other hand, a single 16 meters tall silver maple tree can lose 54 x 4.5 liters of water per hour. If a rough estimate can be made of an average forest in South India, 6500-8000 x 4.5 liters of water / acre / day are lost. The above figures clearly indicate the impact of transpiration, particularly in a country like India, where most of the farmer, depend on the seasonal rainfall for their agriculture. Controlling and economizing of water is one of the most important fields of research in Plant Physiology and Plant Breeding. It is also known that some plants like Potamogeton, Vallisneria (aquatic) have vestigial stomata, which are genetically controlled. If such characters are transferred to crop paints, by either plant breeding or somatic hybridization, it is possible to raise new plants which possess both high yielding and low transpiring attributes.

The magnitude of transpiration by plants, as mentioned before, varies among different species, and also depends upon the environmental conditions. But the most important factor that contributes to this is the structures or surfaces from which transpiration takes place.
Curticular transpiration: Cuticle is the outermost layer of varying thickness found covering the outer surface of leaves and stems etc. This layer is composed of cutin, wax and lipid which are secreted by the underlying cells. These substances are very waxy and hydrophobic in nature. However, depending upon the thickness, little water may percolate or diffuse though these layers, but maximum water is lost through the breaks or openings found in the layer. Nevertheless, the magnitude of transpiration is relatively insignificant.
Lenticular transpiration: In this case, water is lost through lenticels, which are normally found on stems, fruits and pedicels. These structures develop during secondary growth in Dicots. These lenticels are cup like open, structures having a loose tissue called ‘complementary tissue’ with intercellular spaces. The atmospheric air enters into the intercellular spaces of this loose tissue. If the relative humidity of the air is less, then the cells with higher water potentially lose their water into air spaces in the form of water vapors, thus water escapes into outer atmosphere. Though the magnitude of the lenticular transpiration is considerable, still it is insignificant when compared to the stomatal transpiration.
Stomatal transpiration:
Transpiration, in this case, takes place through special pores called stomata. The amount of transpired water through these structures is more than 80-90% of the total transpired water. Hence the structure and the number of these stomata play a significant role in the water.

Most of the stomata are found in leaves, but the presence of these structures on stems, petioles, sepals and petals is not uncommon; the numerical distribution of the stomata in a given leaf can be calculated by stomatal index. Stomatal index is a measure of total number of stomata per total number of epidermal cells in a given unit area. Different plants show different stomatal index. Further the number varies not only from plant to plant, but also varies in different environmental conditions. Basing on stomatal index different stomatal types have been recognized 1) Apple type Mulberry type 2) Potato type, 3) Ota type, 4) Water lily type 5) Potomogeton type.


Efficiency of Stomata: As stomata are small pores, water evaporates efficiently. When the perimeters of all the pores of stomata are taken together and then compared with the total surface area, the former far exceeds the latter. Added to this the water escapes in higher magnitude at perimeters, than the central region of the pores. Hence stomatal apparatus is well suited for higher transpiration rate.
Stomatal apparatus: The stomatal apparatus of monocots and dicots vary in their structure and dimensions. Generally, the stomatal apparatus is derived from epidermal cells. It consists of a pore, and it is surrounded by two kidney shaped epidermal cells called guard cells, which in turn are surrounded by subsidiary cells or accessory cells. The guard cells are also in contact with spongy parenchymatous cells of inner mesophyll. Normally stomata lead into relatively large air spaces. Guard cells in the case of monocots are dumbbell shaped structures with thick walls on both face and thin wall and thin wall at the inflated ends. They are also surrounded by subsidiary cells. The guard cells are unique in having the following features; they have active nucleus; their inner walls are considerably thick and the outer walls are very thin and extensible the ends of the paired guard cells are tightly concerned. There are distinct cytoplasmic strands or plasmodesmata connections between inner spongy parenchymatous cells and guard cells. The most important feature of these cells is the presence of very well developed and photosynthetically active chloroplasts which are totally absent in other epidermal cells. These guard cells are also highly sensitive to the changes in pH, CO2, light, temperature, phytohormones, and water stress.
General mechanism of stomatal transpiration
Water absorbed by the root system is transported upwards through xylem elements by transpiration pull mechanism and reaches the serial parts like leaves. Through the vascular system found in the veins of leaves, the water enters into mesophyll cells. If the relative humidity of the atmosphere present in air spaces is low, water from the surrounding cell surfaces, escapes in the form of water vapors into these spaces.
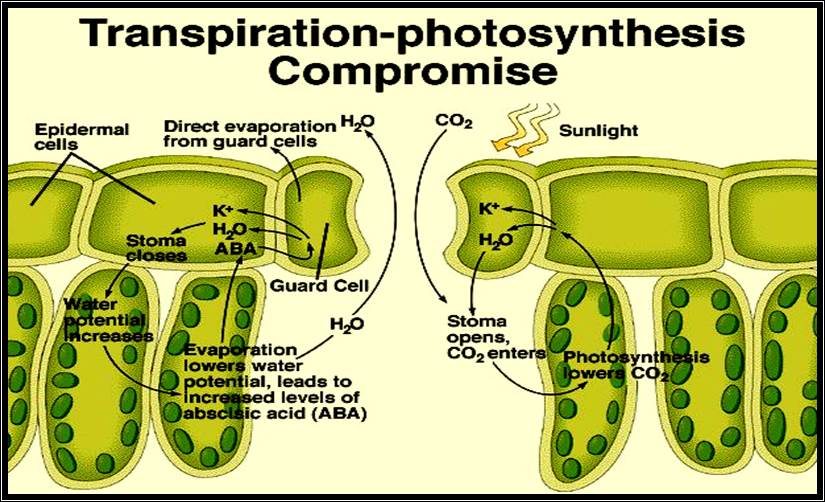
If the stomata are opened, water vapors escape into air, or remain in the spaces if the stomata are closed. Thus stomatal movement plays a pivotal role in regulating the loss of water from the plants.
The mechanism of opening and closing of the stomata:
Though this process is known to Plant Biologists for more than a century, there is no unequivocal explanation which explains the mechanism of opening and closing of
stomata.

This is because of the intrinsic and extrinsic factors which play a major role in the guard cell movements. Nevertheless, plant physiologists have been proposing theories from time to time, but they are either being contradicted or lagging in convincing explanation with reference to some specific experimental observations. Despite its complexity, it is worthy to make an attempt to understand the mechanism of opening and closing of stomata.
It is interesting to know, how stomata respond to various factors and what effects these factors have on guard cells.
1. Stomata open at day time and remain closed during night times. This means that light plays an effective role.
2. Blue and red light of solar electro-magnetic radiation spectrum favors opening which is incidentally the action spectrum of photosynthesis. Other wave lengths of light have no significant effects.
3. Increased CO2 concentration induces the closing, and decreased concentration stimulates the opening of the stomata.
4. Stomata begin to open when the pH of the medium is high (alkaline) and close when pH is low (acidic).
a) During day time Starch disappears in guard cells and at night times Starch accumulates. This feature is contrary to the other mesophyll cells, where Starch disappears at nights and accumulates during day times.
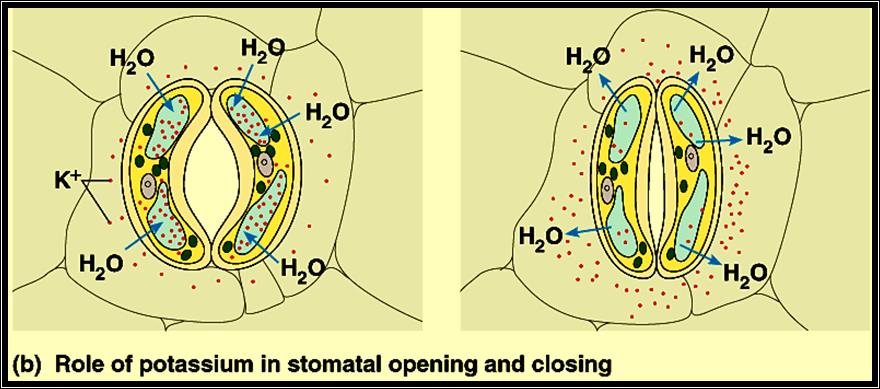
5. Influx of K ions into guard cells induces the opening and efflux of K favors closing
6. Phytohormone like Cytokinin stimulates the guard cells to open and Abscissic Acid (ABA) induces the closing.
7. In succulents like cactus etc., stomata open during nights and close at day times. This is correlated to Carboxylic Acid Metabolism (CAM) where organic acids accumulate during night and the same are decarboxylated at day time. The notable organic acids synthesized are mainly Malate and Glycollate.
8. Glycollate induces the opening of stomata and glycollate oxidation favors the closing of the stomata.
Theories
As explained earlier, the mechanism involved in opening and closing of the stomata are more complex. Inspite of this, it is significant to know how different concepts were developed and their merits and demerits.
1) Turgour Pressure Theory: At the end of the 19th century, plant physiologists believed that, as guard cells contain chloroplasts, they are capable of photosynthesis. Consequently, H2O present in guard cells is used up in the said process; at the same time Glucose is synthesized. Thus, both the steps cause an increase in the DPD of cells. As a result, water from neighboring cells enters into guard cells creating an increased turgour pressure. Hence stomata open and lose water.
At the outset, this presumption is quite attractive, but experimental facts are different. Amount of water consumed during photosynthesis is so low that it cannot have any significant effect on DPD of guard cells, while Glucose synthesized during photosynthesis is a fact, but qualitative analysis shows that glucose does not contribute anything significant towards the DPD increase in guard cells. Thus the theory fails to explain the true mechanism.
2) Starch Hydrolysis theory: Sayre (1926) and Searly (1932) proposed their theory, which are even though different from one another show some similarities. The observations like 1) Presence of starch in guard cells at night and its disappearance during day time, 2) Opening of stomata under high pH condition and closing at low pH and 3) Decrease of CO2 level during day time and increase at night, are very well correlated to each other.
During the day time green plants start synthesizing Glucose by fixing CO2. Thus the concentration of CO2 decreases within the leaves, as well as outside the leaves. This decrease in CO2 concentration raises or increases the pH of the cell sap of guard cells. Due to this change in pH, certain enzymes found in guard cells get activated and start hydrolyzing the Starch (Osmotically inactive) which results in release of Glucose (Osmotically active). This enzymatic conversion causes an increase in the DPD of guard cells. Automatically a DPD gradient is created between the guard cells and neighboring cells which makes the water to diffuse into guard cells. As a result, turgour pressure increases and guard cells bulge and open. The entire process is reversed during night (darkness) by converting osmotically active Glucose to osmotically inactive Starch. This is due to the increased concentration of CO2 because of continued respiration. This theory has got a good support from the discovery of starch phosphorylase enzyme in guard cells by Yung and Tan (1948).
PH 7
Starch + Pi --> Glucose 1 – P
PH 5
Though this theory has some support from the experimental evidences, it is not known whether the same enzyme is involved in the dark conversion of Glucose 1-P to Starch in closing of the stomata, or not. Thus the theory has remained incomplete.
3. Steward’s Theory: Steward (1964) basing on previous observations, modified the theory of Starch hydrolysis. He concurred with the earlier view up to the formation of G-1-P but he express that G-1-P is not osmotically active, and to produce osmotically active molecules, G-1-P has to be converted to Glucose, via phosphoglucomutase and phosphotase action. It is increase of Glucose concentration that causes the DPD gradient which results in the increase the TP of the guard cells and opening of stomata. Further he viewed that closing of the stomata is an active process, for Glucose is converted to Starch through hexokinase (which requires ATP) and starch synthesis activity. The conversion of Glucose to Starch leads to the fall of DPD level causing the guard cells to become flaccid and therefore the stomata close. This theory popular and was widely accepted.
But soon people realized that Steward’s hypothesis was contradicted by certain observations like, a) closure of stomata in the mid-day b) some guard cells are lacking it starch, c) effect of glycollate on opening of stomata. Thus alternate explanations were sought.
1. Zelitch & Kevil’s theory of organic acids: Further studies on the mechanism of opening and closing of stomata were made to relate light, CO2 and pH interactions. Then it was observed that K ions influx and efflux played a significant role in opening and closing of the stomata. Independent studies showed that the phytohormones like Cytokinin induced the opening and ABA favored the closing of stomata. They have also found that Cytokinin stimulated the intake of K ions from the surrounding cells and ABA induced the efflux of K ions. The uptake of K ions was found to be ATP activated. K* / H* + AT Pase pumps found in the plasma-

2. Membranes of guard cells are responsible for the intake of K* ions, which causes the increase in osmotic pressure and increase in DPD. Thus water enters the guard cells from inner cells resulting in the opening. The efflux deflates the guard cells and stomata cells. This phenomenon became more fascinating with the observation that glycollate and malate synthesis in response to cytokinin in chloroplasts and release the same into the cytoplasm provides anion and H* in countering the effect of K* ions uptake. However, ABA reversed the entire process. This observation has been further supported by the finding that slightest environmental stress like increased temperature, deficit of water, causes the synthesis of ABA.

Despite these facts, the problem of malate / glycollate synthesis in the guard cells remained a nagging problem for sometime. A parallel situation in CAM plants was found where malate was accumulated during dark conditions and as a consequence of this, stomata opened at nights and closed during day times, where malate was decarboxylated and utilized in carbohydrate synthesis.
With all these observations, it is still not certain how all the observed metabolism are integrated and regulated in opening and closing of stomata, an over all process is diagrammatically represented. The mechanism of opening and closing of stomata is basically controlled by changes in the osmotic potential of the guard cells. Increased OP sets a DPD gradient between guard cells and neighboring cells, when water from higher energy level diffuses into lower potential found in guard cells and causes increased turgour pressure; thus, guard cells bulge and stomata open. The closing of stomata is mostly a reverse of this process.
As the day sets in, actinic light (blue and red) initiates photosynthesis resulting in the depletion of CO2 in the interior of the leaf, as well as outside. At the same time, light activates Cytokinin mediated molecules which in turn stimulate K/H* ATPase pump. As a result, K* ions are pumped into guard cells and OP increases. Simultaneously the depletion of CO2 activates starch phosphorylase, which utilizing inorganic phosphate hydrolyses the osmotically active Starch into osmotically active G-1 – P then to Glucose; hence the DPD of the guard cells raises. Added to this, the increased accumulation of photosynthetically released oxygen stimulates RUDP – oxygenase to yield Glycollate and PGA. Further, Malate is also synthesized; K* ions, Glycollate and Malate accumulate and cause an increase in OP of the guard cells. Thus, water diffuses in and TP builds up and stomata open. The process is reversed at night probably due to the synthesis of ABA, which causes the decarboxylation of Malate, oxidation of Glycollate and out flow of K*; when these things are happening, continued respiration liberates CO2, and the level of O2 accumulation and pH decreases. As a result, from Glucose, the synthesis of osmotically inactive Starch takes place. Thus, the OP of the guard cells decreases and water potential increases. However, change in the pH (to acidic) stimulates the conversion of Starch to Glucose and Fructose in mesophyll cells, whose OP increases significantly and a DPD gradient is created between mesophyll cells and guard cells. This acts as the motive force for the water to move into mesophyll cells and the guard cells collapse and close the stomata.
Factors
The most important factor that controls the rate of transpiration is the relative humidity of atmosphere i.e., the relative amount of moisture (water) present in the atmosphere. If the relative humidity is higher, the rate of transpiration is lower; on the other hand, if the relative humidity is very low the rate of transpiration will be higher.
Any other factor that affects the relative humidity also affects the rate of transpiration. Dry weather conditions like summer and winter, high velocity of wind, all affect the relative humidity and cause high rate of transpiration. Season, weather, altitude, soil moisture, plant body etc., all affects the rate of transpiration.
In order to check or control the excessive transpiration, plants have devised many morphological and physiological adaptations like the development of thick cuticle, development of multiple epidermal layers, sunken stomata, a mat of waxy hairs, and so on. In some cases, like xerophytes, the leaves themselves are either modified into spines or reduced to scaly leaves, thus they totally prevent the loss of water.
Good effects of Transpiration
Transpiration helps in creating suction pressure, which facilitates the ascent of sap as well as absorption of water and to some extent absorption of minerals. Transpiration keeps the cells in continuous flux. It has cooling effect on the plant body and also helps in the development of good roof system and also helps in the development of good roof system and mechanical tissues. Added to this, it helps in the development of good drainage in the soil.
The effects of Transpiration:
In spite of some beneficial effects of transpiration, it is considered as an unavoidable evil. If transpiration is in excess, it may act as a devil and cause harmful effects like permanent wilting, stunted growth, reduced photosynthetic yield, and possibly in extreme conditions it may cause death also.
